- Your cart is empty
- Continue Shopping
Mouse T cell priming is enhanced by maturation-dependent stiffening of the dendritic cell cortex
- Home
- Publication
- Mouse T cell priming is enhanced by maturation-dependent stiffening of the dendritic cell cortex
Mouse T cell priming is enhanced by maturation-dependent stiffening of the dendritic cell cortex
- elsbizadmin404
- Publication
- Reading Time: < 1 minute
[vc_row][vc_column][vc_column_text]
Abstract
T cell activation by dendritic cells (DCs) involves forces exerted by the T cell actin cytoskeleton, which are opposed by the cortical cytoskeleton of the interacting antigen-presenting cell. During an immune response, DCs undergo a maturation process that optimizes their ability to efficiently prime naïve T cells. Using atomic force microscopy, we find that during maturation, DC cortical stiffness increases via a process that involves actin polymerization. Using stimulatory hydrogels and DCs expressing mutant cytoskeletal proteins, we find that increasing stiffness lowers the agonist dose needed for T cell activation. CD4+ T cells exhibit much more profound stiffness dependency than CD8+ T cells. Finally, stiffness responses are most robust when T cells are stimulated with pMHC rather than anti-CD3ε, consistent with a mechanosensing mechanism involving receptor deformation. Taken together, our data reveal that maturation-associated cytoskeletal changes alter the biophysical properties of DCs, providing mechanical cues that costimulate T cell activation.
[/vc_column_text][vc_column_text]
Learn more:
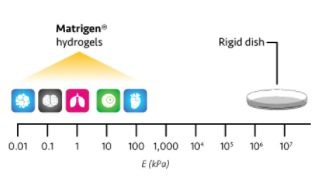
In this study, the authors reported that increasing stiffness lowers the agonist dose needed for T cell activation by using the 96 well plates coated with polyacrylamide hydrogels spanning a stiffness range of 2–25 kPa.
Learn more about Matrigen’s products.
DOI:
10.7554/eLife.55995[/vc_column_text][/vc_column][/vc_row][vc_row][vc_column][vc_separator color=”custom” border_width=”2″ accent_color=”#004a80″][/vc_column][/vc_row]
CONTACT

QUESTIONS IN YOUR MIND?
Connect With Our Technical Specialist.

KNOW WHAT YOU WANT?
Request For A Quotaiton
OTHER BLOGS YOU MIGHT LIKE
HOW CAN WE HELP YOU? Our specialists are to help you find the best product for your application. We will be happy to help you find the right product for the job.

TALK TO A SPECIALIST
Contact our Customer Care, Sales & Scientific Assistance

EMAIL US
Consult and asked questions about our products & services

DOCUMENTATION
Documentation of Technical & Safety Data Sheet, Guides and more..
