Biotium products are distributed only in Singapore and Thailand.
Label your antibody with biotin in just 30 minutes without a purification step. Labeling tolerates many common buffer components including BSA and ascites.
Product Description
Mix-n-Stain™ Biotin Antibody Labeling Kits allow you to biotinylate ≤5 ug up to 100 ug of your antibody in just 30 minutes, with minimal hands-on time and no purification.
Features
- Label 5-20 ug, 20-50 ug, or 50-100 ug Ab
- Less than 30 seconds of hands-on time
- 30 minutes total reaction time
- No purification, 100% recovery
- Compatible with BSA, gelatin, ascites
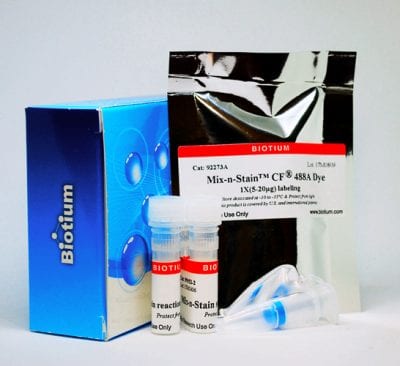
Kit components
- Ultrafiltration spin vial (for antibody concentration or buffer exchange, if needed)
- 10X Mix-n-Stain™ Reaction Buffer
- Lyophilized Reactive Biotin
- Mix-n-Stain™ Storage Buffer
Unrivaled Convenience
Mix-n-Stain™ labeling tolerates common antibody buffer formulations. An ultrafiltration centrifuge vial is provided to quickly remove interfering substances like glycerol, or to concentrate your antibody. With a slight modification in protocol, antibodies can be labeled in the presence of BSA, gelatin, or ascites fluid. The presence of other proteins like BSA or gelatin in the labeling reaction has minimal effect on background fluorescence, because any labeled non-antibody proteins readily wash away during immunofluorescence staining.
Choose the Right Labeling Kit for Your Antibody
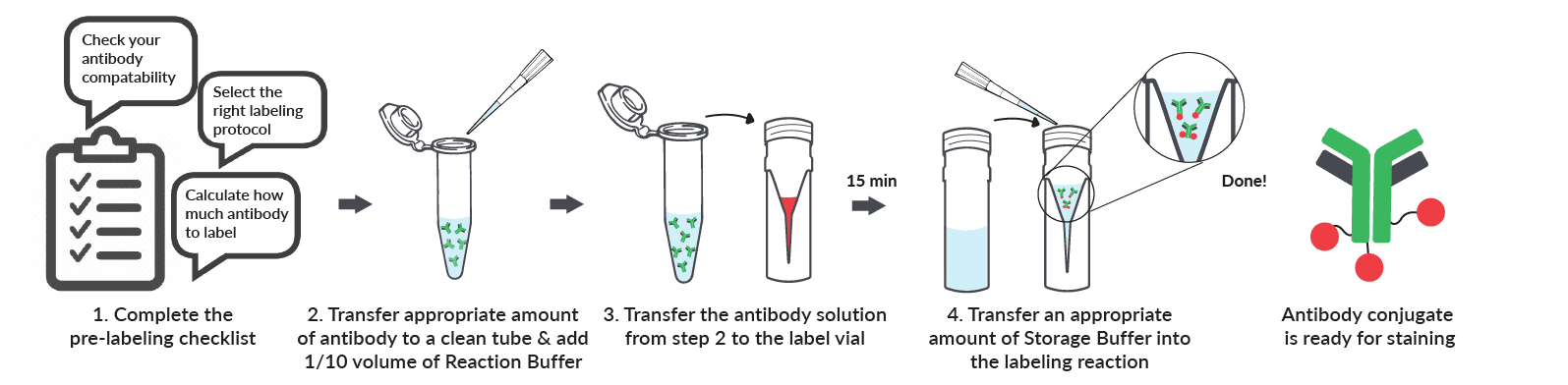
Mix-n-Stain™ Antibody Labeling Kits are very simple to use (see the workflow overview above). But before you begin, you must check that your antibody meets the compatibility requirements for labeling, and choose the right labeling protocol. Download the updated and expanded Product Protocol for a labeling check list to help you select the right kit size and labeling protocol for your antibody. Also see our Kit Compatibility and Protocol Selection Flowchart.
We offer Mix-n-Stain™ Kits for labeling antibodies, nanobodies, and small ligands with a wide selection of fluorescent dyes, enzymes, and other labels. See our full selection of Mix-n-Stain™ Antibody Labeling Kits below.
Mix-n-Stain™ Product Lines
| Product Name |
Label Options |
Labeling Scale |
Labeling Time |
Features |
|---|---|---|---|---|
| Mix-n-Stain™ CF® Dye Antibody Labeling Kits |
CF® Dyes | ≤5-20 ug IgG 20-50 ug IgG 50-100 ug IgG |
~ 30 min. | • Rapid, simple labeling
• No purification • Tolerates BSA & other additives • Dyes for super-resolution, spectral flow, & NIR detection |
| Mix-n-Stain™ FITC Antibody Labeling Kits |
Fluorescein (FITC) | |||
| Mix-n-Stain™ Cyanine Dye Antibody Labeling Kits |
Cyanine 555 (Cy®3) Cyanine 647 (Cy®5) |
|||
| Mix-n-Stain™ Biotin Antibody Labeling Kits |
Biotin | |||
| Mix-n-Stain™ Digoxigenin Antibody Labeling Kits |
Digoxygenin (DIG) | |||
| Mix-n-Stain™ DNP Antibody Labeling Kits |
Dinitrophenol (DNP) | |||
| Mix-n-Stain™ Fluorescent Protein & Tandem Dye Antibody Labeling Kits |
APC R-PE PerCP Tandem Dyes |
20-50 ug IgG 50-100 ug IgG |
~ 4 hours | • Fast, simple labeling
• Minimal hands-on time • No purification |
| Mix-n-Stain™ Enzyme Antibody Labeling Kits |
HRP AP GOx |
HRP: 10-20 ug IgG 25-50 ug IgG 50-100 ug IgGAP or GOx: 25-50 ug IgG 50-100 ug IgG |
~ 2-3 hours | |
| Mix-n-Stain™ Maxi Antibody Labeling Kits |
CF® Dyes Cy® Dyes |
1 mg IgG | ~ 30 min. | |
| Mix-n-Stain™ Nanobody Labeling Kits |
CF® Dyes | 5-20 ug Nanobody® 20-50 ug Nanobody® |
~ 30 min. | • Optimized for Nanobodies®
• Tolerates BSA & other additives |
| Mix-n-Stain™ Small Ligand Labeling Kits |
CF® Dyes | 0.1 umol small ligand |
~ 30 min. | • Label SNAP®, CLIP™, HaloTag® & other small ligands
• Dye options for surface or intracellular targets |
| CF® Dye & Biotin SE Protein Labeling Kits |
CF® Dyes Biotin |
3 x 1 mg protein |
~ 2 hours | • Dyes, buffers, & spin vials for labeling + purification |
Note: Kits are not recommended for labeling IgM antibodies. Cy dye is a registered trademark of GE Healthcare.