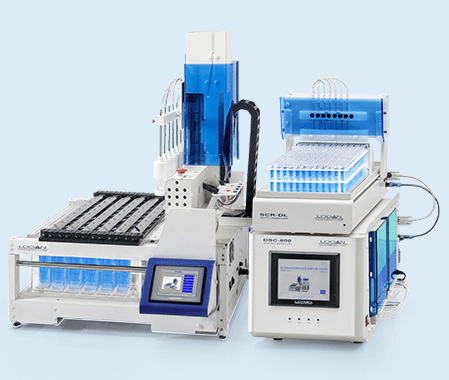
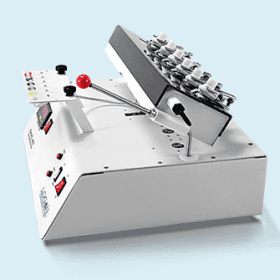
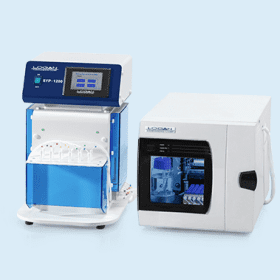
SYSTEM ADR III-7 automated release rate sampling system is suitable for USP apparatus 3 and USP apparatus 7 drug release rate testing. It contains a DISSO III-7 release rate tester, a DSC-800 system controller, a SYP–8L–10mL and a SCR-DL sample collector.
Application:
USP apparatus 3 is used for dissolution testing for control-release drugs, enteric soluble drugs, soft capsules, and chewable drugs.
USP apparatus 7 is used for dissolution testing for transdermal patches, implants, stents, stem and balloons.
Features:
- Switchable between USP apparatus 3 and USP apparatus 7 drug release rate testing
- Unique vessel covers design to prevent evaporation and media contamination
- Automated sampling system for increased efficiency
- Multiple sampling intervals and adjustable dip speed for flexible dissolution methods
- Media replacement as standard configuration to ensure sink condition
- One piece molded clear water bath for easy observation
- Adjustable stroke length suitable for dissolution studies
- Data audit tracking function available and it complies to FDA 21 CFR Part 11 requirements
Extended functions:
- Compatible with camera system to record drug release process
- SYSTEM ADR III-7 is compatible with UV or HPLC. It can perform online dissolution analysis
- Compatible with PERMETRO system to distinguish the permeation between generic drugs and original drugs, which can exist as a pre-BE study