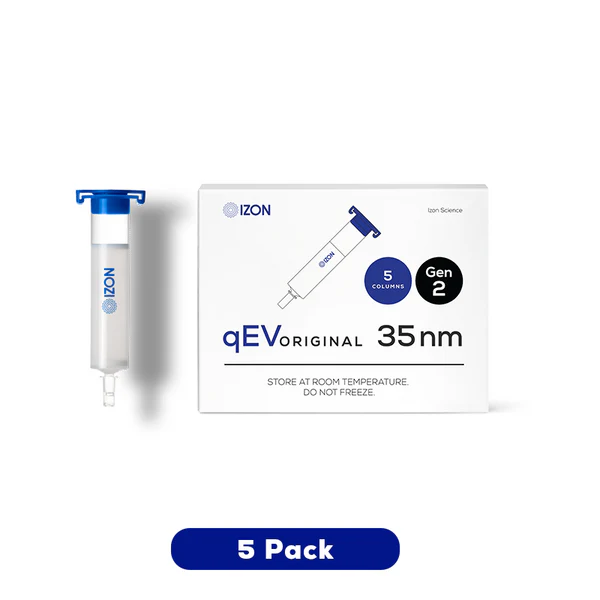
Izon qEV SEC Isolation Columns
Add to cart
qEV provides the most simple and rapid isolation of EV/exosomes. With just 15 minutes, you can easily obtain a highly purified sample of intact exosomes!
Our isolation and measurement techniques bring reproducibility and precision to the extracellular vesicle (EV) field. Together, our qEV Isolation and TRPS measurement technology enable exosomes, microvesicles and other EVs to be isolated prior to size and concentration measurement.
qEV columns harness principles of size exclusion chromatography to provide an effective approach to EV isolation, and when paired with the Automatic Fraction Collector (AFC), streamline and standardise your EV isolation workflow. Tunable resistive pulse sensing (TRPS) is a single-particle measurement technique that utilises the Coulter principle to provide precise, high-resolution measurements on a particle-by-particle basis.
Our qEV isolation platform provides reproducible and efficient separation solutions for fundamental research, and a scalable and standardisable approach for developing EVs in diagnostic, therapeutic, and cosmetic. The qEV columns can also be used for isolating highly pure samples of viruses and virus-like particles for research, diagnostics, monitoring therapeutic response, vaccine development, and gene therapy!
Product highlights:
- No carryover: The qEVsingle is the smallest of the qEV range and the only column designed for single-use
- Precision isolation: Isolate exosomes and other EVs from biofluids or cell culture media with high purity. Available in different columns optimised for different sample loading volumes.
- High purity: Remove soluble proteins effectively to obtain a highly purified EV isolate
- AFC-ready: Fully compatible with the Automatic Fraction Collector (AFC) for efficient and reproducible isolation for all Izon columns, except for qEV100.
- Universal compatibility: Can be easily adapted to automated chromatography systems using Leur Lock hose barb fittings for the following columns, qEV2, qEV10, and qEV100.
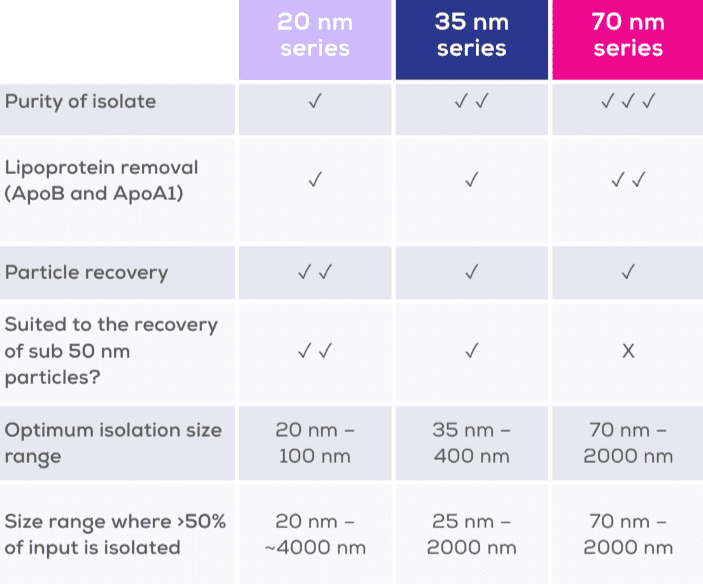
- Choose your resin series: Delve into more smaller particles with the 20 nm range, isolate small EVs with the 35 nm Gen 2 series, or opt for 70 nm Gen 2 columns for greater ApoB removal. Expect longer lead times for the 20 nm range.
- Quality assurance: Izon products are designed and manufactured under a quality system certified to ISO 13485:2016.
How qEV Isolation Columns Work
qEV columns use principles of size exclusion chromatography (SEC) to separate exosomes and other extracellular vesicles (EVs) from contaminating particles, such as proteins and lipoproteins.
The molecules are separated by their size as they pass through a column consisting of porous resin particles. In qEV isolation, larger particles are unable to enter pores in the resin, and instead flow through the resin relatively quickly. In contrast, particles smaller than the lower end of the isolation range (20, 35 nm or 70 nm) enter pores in the resin, which slows their journey. Through this size-based separation, EVs and soluble protein are separated to a high degree of resolution, and a purified volume can be obtained.
The New qEV Gen 2
The qEV Gen 2 range is comprised of Izon’s most advanced size exclusion chromatography columns. To achieve the best possible results, qEV Gen 2 columns are made from a high-performance resin developed specifically for the isolation of extracellular vesicles (EVs). Spanning six column sizes, the complete range is comprised of columns optimised for different sample loading volumes. This diversity enables qEV Gen 2 columns to provide a strong foundation for EV isolation across diagnostics, therapeutics, and fundamental research.
GMP-Ready Columns:
Izon’s qEV columns are used by global biopharmaceutical companies working with extracellular vesicles, and we are prepared to assist with your regulatory requirements. We have implemented a number of measures in qEV column production, to ensure the necessary protocols and validation assays are in place. Each batch of GMP-ready columns is subject to bioburden and endotoxin testing, with the results compared against defined criteria for release.

 简体中文
简体中文 繁體中文
繁體中文 English
English 한국어
한국어 ไทย
ไทย Tiếng Việt
Tiếng Việt