Product Description
1. Product Features
- · A validation product for biological mycoplasma as well as for mycoplasma contamination in the production cell strain
- · Contains the main contaminants of the cell culture including M. orale, M. hyorhinis, M. arginini, M. fermentans, M. hominis, Acholeplasma laidlawii, allowing all mycoplasma detection of M. pneumoniae, M. salivarium, M. genitalium, M. penentrans and ureaplasma
- · The primer binds to the highly conserved 16s rRNA coding region
- · DNA of eukaryotes and other bacterial species are not detected
- · PCR performance monitoring is possible with the application of internal amplification control (IAC)
- · Optimized PCR Premix provided for easy use
- · General PCR technique applied
- · Main strand DNA preparation using cell culture solution or commercial DNA extraction kit
- · Sensitivity: 1 ~ 10 fg/reaction
- · Result confirmation within 1–2 hr
- · Passed the Korea Ministry of Food and Drug Safety (MFDS), European Pharmacopoeia (EP), and Japanese Pharmacopoeia (JP) standards
- · UDG system application: Prevention of carry-over contamination
2. Product results
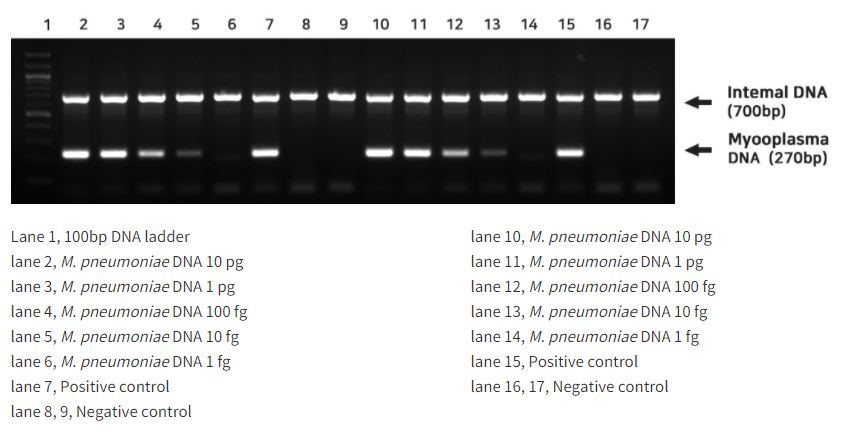
An approximate of 250–300 bp DNA band can be observed at mycoplasma contamination. Furthermore, when PCR reaction is performed appropriately, an internal DNA band of an approximately 700 bp can be identified.
3. Product type
| Type | Product name | Cat. No. | Size |
|---|---|---|---|
| Mycoplasma test (QC) | HiSense™ Mycoplasma PCR Detection Kit | HD-50 | 50 Tests |
| HD-100 | 100 Tests |
4. User menual