Summary
Traceability is paramount for ensuring reproducibility with FBS in cancer cell culture research. As exemplified by the ISIA Traceability Program, adhering to stringent quality standards and meticulous documentation procedures safeguards the integrity of serum products. While cost can be a factor, researchers should prioritise the credibility and reliability of their FBS. Some products may seem like a cheaper alternative, but sacrificing quality can jeopardise research outcomes. Investing in traceable, high-quality serum ensures consistency and trustworthiness in experimental results, ultimately advancing the reliability and validity of cancer research. Traceability safeguards the integrity of scientific inquiry and paves the way for meaningful progress in understanding and combating cancer.
Reproducibility is a cornerstone of progress in cancer research, and its pursuit hinges on the meticulous use of foetal bovine serum (FBS) in cell culture methodologies. Effective use of FBS in cell cultures relies on its traceability, which ensures authenticity and consistency throughout research endeavours. To ensure reliable outcomes from these experiments, traceability of FBS is crucial.
Why is The Traceability of FBS Important in Cancer Research?
FBS is a commonly used supplement in cell culture media due to its rich source of growth factors, hormones, and other nutrients essential for cell growth and proliferation. In cancer research and drug screening, traceability in FBS is crucial for several reasons:
- Quality Control: Traceability ensures that the FBS used in cancer research meets stringent quality standards. FBS quality can vary depending on factors such as the source, processing methods, and storage conditions. Traceability allows researchers to track the origin of FBS batches, ensuring that they are obtained from reputable suppliers and manufactured using standardised protocols. This reduces the risk of variability in experimental outcomes due to differences in FBS quality.
- Consistency in Experimental Conditions: Consistent FBS quality is essential for maintaining reproducible experimental conditions in cancer research, especially in drug screening studies. Variations in FBS composition can influence cell behaviour and drug response, potentially leading to unreliable results. Traceability enables researchers to select FBS batches with known characteristics, ensuring consistency across experiments and facilitating more accurate comparisons between different treatment groups.
- Minimisation of Contamination Risks: FBS contamination can compromise experimental integrity and lead to inaccurate results in cancer research. Traceability allows researchers to identify and trace the source of FBS contamination, enabling timely interventions to mitigate its impact on experimental outcomes. By selecting traceable FBS batches with a documented history of quality control measures, researchers can minimise the risk of contamination-related issues and maintain the reliability of their research findings.
- Transparency and Data Interpretation: Traceability promotes transparency in cancer research by providing researchers with detailed information about the origin, processing, and testing of FBS batches. This transparency allows researchers to make informed decisions about the suitability of FBS for their experiments and interpret their data accurately. By understanding the specific characteristics of the FBS used in their studies, researchers can better evaluate the relevance of their findings and draw meaningful conclusions about the efficacy of cancer drugs and treatments.
- Compliance with Regulatory Standards: Many regulatory agencies require documentation of the origin and quality of biological materials used in research, including FBS. Traceability ensures compliance with regulatory standards by providing researchers with the necessary documentation to support their experimental protocols and data interpretation. By maintaining comprehensive records of FBS sourcing and handling practices, researchers can demonstrate adherence to regulatory guidelines and enhance the credibility of their research outcomes.
The Importance of FBS Authenticity
Concerns about the quality and sourcing of FBS have plagued research for years. These concerns include:
- Contamination: Potential contamination with adventitious agents or undisclosed additives can significantly skew experimental results.
- Mislabelled FBS: Instances of mislabelled FBS origin, where serum is falsely attributed to a specific region or supplier, compromise reproducibility and regulatory compliance.
A notable example involved smuggled South American serum being rebranded and sold as Australian origin. This highlights the need for vigilance in ensuring FBS authenticity.
Traceability: The Key to Authenticity
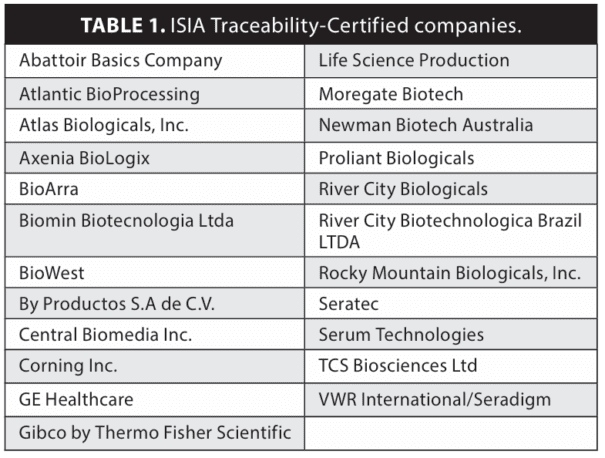
Maintaining traceability throughout the FBS supply chain is crucial for upholding research integrity and reproducibility. Initiatives like the International Serum Industry Association (ISIA) traceability certification program guarantee the quality and origin of FBS.
International Serum Industry Association (ISIA) traceability certification
The International Serum Industry Association (ISIA), founded in 2006, represents the global serum industry, producing and distributing various animal serum types, including FBS. ISIA’s key initiative establishes standards, guidelines, and the Traceability Certification program to ensure serum quality, traceability, and compliance.
ISIA Traceability Certification involves rigorous documentation, quality control, and auditing, ensuring proper sourcing, handling, storage, and origin testing. Manufacturers and suppliers undergo certification processes, enhancing transparency and supply chain traceability.
ISIA Traceability Certification signifies manufacturers’ commitment to quality and traceability, providing researchers and end-users confidence in serum reliability. It’s particularly crucial for biopharmaceutical companies, ensuring materials align with specifications and maintaining integrity, especially considering past transparency issues. This certification serves as an additional assurance, confirming material authenticity and aligning with desired standards, enhancing confidence in purchased materials.
The Importance of Serum Origin
The origin of serum is critical for several reasons:
- Animal Health: The health of the animals from which serum is collected impacts its suitability for research.
- Regulatory Compliance: Different countries have varying regulations for importing serum, based on the animal health status of the exporting nation.
- Research Integrity: Reliable serum is essential for ensuring the reproducibility and accuracy of research results.
Import regulations are established by organizations like the World Organization for Animal Health (OIE), which tracks and reports global animal diseases.
BSE and Serum Origin: A Mitigated Risk
Bovine Spongiform Encephalopathy (BSE), or mad cow disease, was a major concern after its discovery in the 1980s. However, thanks to collaborative efforts by the OIE and regulatory bodies, the prevalence of BSE has significantly decreased. Many countries have been classified as having “Negligible BSE risk” or “Controlled BSE risk” under international animal health guidelines. Importantly, the risk of BSE transmission through serum has been negligible for many years, regardless of origin.
Focus on Quality, Not Just Origin
While serum origin is important, it shouldn’t be the sole factor determining quality. Traceability ensures that serum meets all regulatory and industry standards, not just geographically specific ones. High-quality serum for reproducible research can come from any country that adheres to these strict requirements.
Biowest: Atlantis Bioscience’s Choice for Reliable Results Through Traceable Serum Products
In cancer research, ensuring reproducible results is paramount. FBS is a critical component of cell culture, but its inherent variability can impact experiments. Biowest, a leading supplier of serum products, addresses this challenge by prioritising traceability and quality control.
Biowest offers a diverse range of FBS origins, including sources from South America, the European Union (EU), and those approved by the United States Department of Agriculture (USDA). This variety caters to researchers’ specific needs while adhering to rigorous standards. Biowest’s commitment is evident through their certifications:
- International Serum Industry Association (ISIA): Guarantees adherence to quality and traceability guidelines.
- Certificates of Suitability (CEP) from the European Directorate for the Quality of Medicines (EDQM): Ensures minimal risk of Bovine Spongiform Encephalopathy (BSE).
- European Pharmacopeia (EP) compliance: Meets stringent purity and safety criteria.
Beyond certifications, Biowest prioritises meticulous traceability across its entire process. They meticulously track the origin, processing, and distribution of each FBS batch. This allows researchers to:
- Maintain consistent experimental conditions: Crucial for reliable comparisons in drug screening studies.
- Minimise contamination risks: Traceability facilitates identifying and mitigating potential contamination issues.
- Ensure transparency and data interpretation: Detailed documentation like certificates of analysis and origin allows researchers to understand the specific characteristics of their FBS and interpret data accurately.
Biowest’s dedication to quality, traceability, and compliance ensures researchers receive reliable and consistent serum products. This, in turn, empowers researchers to contribute to advancements in cancer research with confidence in the reproducibility of their findings.
References
Hawkes, P.W. Fetal bovine serum: geographic origin and regulatory relevance of viral contamination. Bioresour. Bioprocess. 2, 34 (2015). https://doi.org/10.1186/s40643-015-0063-7
Versteegen R, Shatova O, Lind S, Linterman K. Testing for geographic origin of fetal bovine serum. BioProcess J, 2019; 18. https://doi.org/10.12665/J18OA.Versteegen