Imagine months of meticulous cell culture work, only to discover your results are worthless due to a silent invader: Mycoplasma. Cell culture has revolutionised biomedical research, allowing scientists to study cellular behaviour and develop therapeutic strategies in a controlled setting. However, maintaining contamination-free cell cultures is essential for reliable and reproducible results.
Among the various contaminants, mycoplasma contamination stands out due to its prevalence and impact on cell health and experimental outcomes. It significantly threatens all cell line-based research and cell-derived biologics production facilities. In this blog, we delve into the basics of mycoplasma contamination in cell culture and discuss strategies for prevention and detection.
What are Mycoplasma?
Mycoplasmas are stealthy invaders in the world of cell culture. These parasitic bacteria are microscopic (0.3 to 0.8 µm) – even smaller than the filters commonly used to sterilise cell culture media and reagents (typically 0.45 µm). This minuscule size allows them to slip through these filters undetected.
But their troublemaking doesn’t stop there. Unlike most bacteria, mycoplasmas lack a cell wall, making them resistant to common antibiotics like penicillin and streptomycin. Additionally, their small size makes them difficult to spot under a conventional light microscope.
Over 200 Mycoplasma species exist, but six species are the primary culprits behind cell culture contamination. These culprits come from various sources, including bovine (M. arginini, Acholeplasma laidlawii), swine (M. hyorhinis), and humans (M. orale, M. hominis, M. fermentans).
The ability to infect a wide range of hosts, including humans, animals, and even plants, makes mycoplasmas a common and persistent threat in cell culture labs.
The Impact of Mycoplasmas Contamination
Mycoplasma contamination presents a significant challenge in cell culture due to its often subtle nature. Unlike readily identifiable bacterial contaminants that cause turbidity in the culture medium, mycoplasmas are stealthy pathogens. These parasitic bacteria act as metabolic competitors within the host cell environment. They compete for essential nutrients present in the culture media, thereby compromising the growth and viability of the cultured cells.
Mycoplasma does not change the pH or turbidity of the culture medium, but it affects changes in cell morphology, growth rate, metabolism, and functions. This disruption in cellular homeostasis can lead to a cascade of detrimental effects, including:
- Cellular Dysfunction: Mycoplasma contamination can alter gene expression and cellular signalling pathways, ultimately leading to impaired cellular function.
- Genomic Instability: The presence of mycoplasmas can induce DNA damage and even chromosomal aberrations within host cells.
The ramifications of mycoplasma contamination extend beyond the immediate loss of cell viability and compromised experimental data. Resources such as time, personnel effort, and financial investment in contaminated cultures are wasted. More critically, research based on mycoplasma-infected cells can yield inaccurate or misleading results, potentially impacting the validity of scientific publications and downstream applications, particularly in biopharmaceutical production.
What Causes Mycoplasma Contamination?
Mycoplasma contamination is a constant threat in cell culture due to its ability to infiltrate cultures through various pathways. Here are the primary culprits:
- Lab Personnel: Humans are surprisingly significant contributors to mycoplasma contamination. Activities like talking, coughing, or sneezing near cultures can generate aerosols that carry these tiny organisms.
- Contaminated Supplies: Mycoplasmas can lurk in various cell culture supplies if not handled appropriately. This includes foetal bovine serum (FBS), cell culture media, and even incubator water baths if not disinfected regularly.
- Cross-contamination: This occurs when mycoplasmas spread from infected cultures to uncontaminated ones. Sharing pipettes or working with multiple cell lines nearby can increase this risk. Furthermore, some labs may unknowingly receive contaminated cell lines due to a lack of routine mycoplasma testing.
- External Sources: While less frequent, mycoplasmas can occasionally enter the lab environment from external sources like dust or airborne particles.
 How to prevent mycoplasma contamination?
How to prevent mycoplasma contamination?
‘Management of mycoplasma contamination must be the central focus for any cell culture lab contamination or quality control program’, Lincoln, CK et al. Mycoplasma contamination is a constant threat, but with a multi-pronged approach, you can significantly reduce the risk. Here are key strategies for preventing mycoplasma in your cell culture experiments:
1. The Aseptic Technique is Paramount
Maintaining a clean lab space is the most basic way to prevent contamination from occurring. Always prioritise the aseptic technique when handling cell cultures. This includes frequent handwashing, and using sterile technique for all procedures. Regularly disinfect incubators, tissue culture hoods, and laboratory surfaces with appropriate disinfectants effective against mycoplasmas. Remember, mycoplasmas can hitch a ride on aerosols generated from talking or sneezing near cultures, so minimise talking in the cell culture hood.
But here’s the thing: while 70% ethanol is effective against some bacteria and fungi, it often falls short against mycoplasma contamination. These tiny buggers can hide within biofilms and even survive in desiccated conditions!
For a more robust decontamination strategy, consider using a broad-spectrum disinfectant specifically formulated to combat mycoplasma contamination.
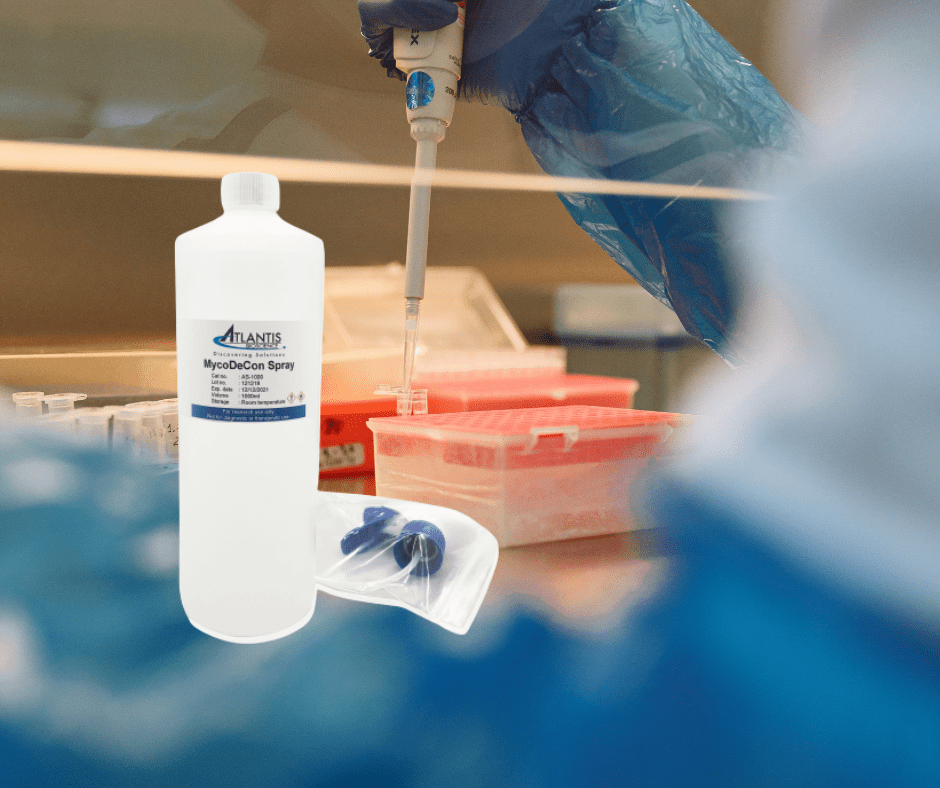
Atlantis Bioscience’s MycoDecon Spray offers a superior alternative disinfectant to inhibit mycoplasma growth. This product kills not only mycoplasma, but also a wide variety of fungi, bacteria, and viruses quickly and effectively. It is compatible with all common workspaces even in an incubator, storage box, or liquid nitrogen tank. It is formulated with gentle yet powerful ingredients that won’t harm your precious cell cultures and is the preferred choice for many cell culture labs in Singapore and Korea.
2. Minimise Cross-Contamination
Working with just one cell line at a time and dedicating separate materials for each culture helps prevent the spread of mycoplasmas. Don’t reuse pipettes between cultures, and consider using dedicated equipment for heavily trafficked cell lines.
3. Routine Screening: Catch Mycoplasma Before it Wreaks Havoc
Regular monitoring for mycoplasma contamination is vital for early detection and swift action. Many laboratories across the world neglect the importance of regular mycoplasma testing, resulting in minimal or no changes in mycoplasma infection rates even with advanced detection methods available. The recommended interval for routine tests is between 2 weeks to 3 months depending on the cells that you culture in the lab.
There are several methods to detect mycoplasma contamination. These include direct culture, indirect culture, and PCR-based detection.
The direct culture method involves culturing the samples on agar plates for colony growth. Formation of colonies indicates the presence of mycoplasma, however, this method is time-consuming (up to 28 days!) and not able to detect non-cultivable mycoplasma.
The indirect traditional fluorescence method is faster than the direct culture. This method uses DNA-binding fluorescent stains, such as Hoechst 33258 or DAPI, to stain the mycoplasma DNA. The appearance of filamentous structures within cells indicates a positive finding. However, it has lower sensitivity and can be subjective for interpretation. Hence, PCR analysis is recommended for routine mycoplasma screenings. This method is sensitive and rapid (results are obtained within hours) and detects cultivable and non-cultivable mycoplasma species.
If your lab is part of the statistics that do not do routine mycoplasma labs, it is recommended that you run a rapid detection test the moment you suspect that your cell culture might be contaminated.
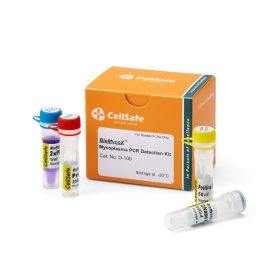
Atlantis Bioscience’s BioMycoX® Mycoplasma PCR Detection Kit provides a rapid and reliable way to screen your cultures. This easy-to-use PCR-based kit targets a specific region of the mycoplasma genome – the 16S ribosomal RNA (rRNA) gene. This highly conserved region allows the kit to detect almost all kinds of mycoplasma species, including the top six species most likely to affect cell cultures: M. arginini, M. fermentans, M. hominis, M. hyorhinis, M. orale, and A. laidlawii.
With BioMycoX®, you can gain peace of mind knowing your cell cultures are free from mycoplasma contamination. This is especially important as many scientific journals require cell lines to be tested for mycoplasmas before publication. BioMycoX® allows you to meet these requirements with confidence and ensure the integrity of your research data.
4. Quarantine Newcomers
Newly acquired or established cell lines should be quarantined and rigorously tested for mycoplasma contamination before integrating them into your research. This quarantine period allows for verification of their mycoplasma status before they come into contact with your existing cultures.
If mycoplasma has already been contaminated, what should I do next?
If your prevention methods still result in mycoplasma contamination, you’ll need to use antibiotics to treat your cultures. Since mycoplasma does not have a cell wall, antibiotics like penicillin that we normally use in the laboratory are not compatible. So, what can effectively kill mycoplasma?
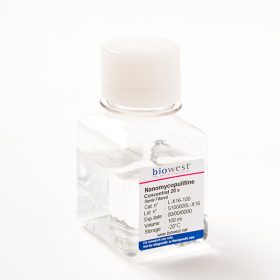
Discover Nanomycopulitine® an antibiotic for mycoplasma elimination that does not target the cell wall, but targets the anabolic pathway of mycoplasma instead. The solution also can eliminate other bacteria, both gram-positive and negative without affecting the metabolism and enzymatic functions of the cells. It has been proven that it is biodegradable, so it is safe for humans and the environment. Plus, Nanomycopulitine® provides long-lasting protection against re-contamination.
Mycoplasma contamination is a precarious problem in cell culture laboratories. We can’t eliminate cell culture contamination but we can reduce the damage caused by mycoplasma with better control. It is recommended that you test routinely to prevent the spread of them. However, once it is contaminated in your culture, do not fret. Our range of prevention and decontamination solutions will be available to give you the necessary support for your translational research.
Don’t let mycoplasma derail your research. Partner with Atlantis Bioscience for a comprehensive approach to maintaining mycoplasma-free cell cultures!
References:
-
- Dennert K, Kumar R. Traceability Methods for Cell Line Authentication and Mycoplasma Detection. SLAS Technol. 2021;26(6):630-636. doi:10.1177/24726303211030290
-
- Drexler HG, Uphoff CC. Mycoplasma contamination of cell cultures: Incidence, sources, effects, detection, elimination, prevention. Cytotechnology. 2002 Jul;39(2):75-90. doi: 10.1023/A:1022913015916.
-
- Geraghty, R., Capes-Davis, A., Davis, J. et al. Guidelines for the use of cell lines in biomedical research. Br J Cancer 111, 1021–1046 (2014). https://doi.org/10.1038/bjc.2014.166
-
- Nikfarjam L, Farzaneh P. Prevention and detection of Mycoplasma contamination in cell culture. Cell J. 2012 Winter;13(4):203-12. Epub 2011 Dec 22.