- Your cart is empty
- Continue Shopping
MSCs vs. iPSCs: Navigating the Landscape of Stem Cell Therapies
MSCs vs. iPSCs: Navigating the Landscape of Stem Cell Therapies
- Atlantis Bioscience
- Blog
- Reading Time: 5 minutes
Stem cell research is rapidly evolving, offering exciting possibilities for treating a wide range of diseases and injuries. Two prominent players in this field are Mesenchymal Stem Cells (MSCs) and Induced Pluripotent Stem Cells (iPSCs). While both hold immense promise, their origin, characteristics, and potential applications differ. Let’s explore these fascinating cell types and see how they contribute to the future of regenerative medicine.
The Ethical Landscape:
It’s important to acknowledge the ethical considerations surrounding stem cell research. Traditionally, research relied on embryonic stem cells (ESCs), raising ethical concerns about embryo destruction. MSCs and iPSCs offer alternative solutions, potentially bypassing these concerns.
Mesenchymal Stem Cells (MSCs):
Derived from various adult tissues such as bone marrow, adipose tissue, and umbilical cord, MSCs have garnered attention for their regenerative properties and immunomodulatory effects. With the ability to differentiate into osteoblasts, chondrocytes, and adipocytes, MSCs offer promise in tissue repair and inflammation modulation.
Key Attributes of MSCs:
1. Tissue Repair:
- Multipotent Differentiation: While not pluripotent, MSCs possess multipotency, enabling them to differentiate into a defined set of specialised cell types within a specific lineage. Bone marrow-derived MSCs, for example, can differentiate into osteoblasts (bone cells), chondrocytes (cartilage cells), and adipocytes (fat cells). This targeted differentiation capacity allows MSCs to directly contribute to tissue repair.
- Paracrine Orchestration: Beyond direct cell replacement, MSCs exert a potent influence through their paracrine effect. They secrete a complex cocktail of growth factors, cytokines, and other signalling molecules. This molecular orchestra creates a supportive microenvironment, stimulating the body’s intrinsic healing mechanisms. These factors promote cell proliferation, migration, and differentiation of resident stem cells, ultimately facilitating tissue regeneration.
2. Immunomodulation:
- Masters of Immunological Equilibrium: MSCs possess remarkable immunomodulatory properties. They can interact with and influence the activity of diverse immune cells, including T-cells, macrophages, and natural killer cells. This influence promotes an anti-inflammatory response, which is crucial for mitigating the detrimental effects of an overactive immune system. This makes MSCs potential candidates for treating autoimmune diseases, where the body attacks its own tissues, and graft-versus-host disease (GVHD), a life-threatening complication following bone marrow transplantation.
- Mechanisms of Immunomodulatory Action: The precise mechanisms by which MSCs modulate the immune system are still under investigation. However, some key pathways include:
- Suppressing the proliferation of pro-inflammatory T-cells
- Inducing the production of regulatory T-cells (Tregs) that promote immune tolerance
- Reducing the activity of antigen-presenting cells, which play a critical role in activating immune responses
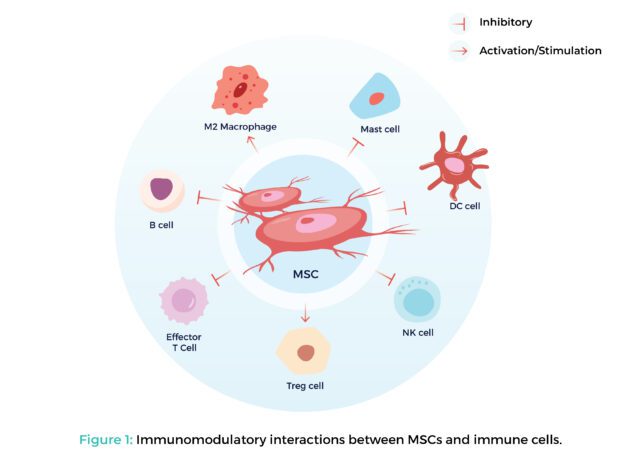
Figure 1: Immunomodulatory interactions between MSCs and immune cells.
3. Minimal Risk of Tumorigenicity:
- Limited Self-Renewal Capacity: Compared to pluripotent stem cells, MSCs exhibit a limited capacity for self-renewal. This translates to a finite number of cell divisions before they cease proliferation. This limited self-renewal potential significantly reduces the risk of uncontrolled cell growth and tumour formation after transplantation.
- Rigorous Safety Protocols: Despite the lower risk, researchers prioritise safety. Stringent quality control measures and extensive pre-clinical testing are essential to ensure the safe and efficacious application of MSC-based therapies.
Induced Pluripotent Stem Cells (iPSCs):
iPSCs, generated through cellular reprogramming of adult somatic cells, hold the allure of pluripotency and patient specificity. While still in earlier development stages, with the capacity to differentiate into virtually any cell type in the body, iPSCs offer unparalleled opportunities for disease modelling, drug discovery, and personalised medicine.
Key Attributes of iPSCs:
1. Pluripotency:
- Cellular Chameleons: Unlike MSCs, iPSCs possess the remarkable ability of pluripotency. This means they have the potential to differentiate into any cell type found in the adult human body – muscle cells, neurons, skin cells, and more. This mirrors the developmental potential of embryonic stem cells (ESCs) but avoids the ethical concerns surrounding their use.
- Unlocking Therapeutic Possibilities: The pluripotency of iPSCs allows scientists to generate specific cell types relevant to various diseases. For example, iPSCs derived from a patient with a neurological disorder can be differentiated into neurons harbouring the same genetic mutation. This opens doors for studying disease mechanisms and developing targeted therapies.
2. Disease Modelling:
- Patient-Specific Cell Models: The ability to generate iPSCs from a patient’s own cells is a game-changer for disease modelling. These patient-derived iPSCs can be differentiated into the specific cell type affected by the disease, creating a personalised cell model. This model allows researchers to study the disease process in a controlled laboratory setting, mimicking the patient’s unique genetic makeup.
- Unveiling Disease Mechanisms: By studying patient-derived iPSC models, researchers can gain valuable insights into the molecular and cellular mechanisms underlying various diseases. This knowledge can lead to the identification of novel therapeutic targets and the development of more effective treatment strategies.
3. Personalised Therapies
- Minimising Immune Rejection: A significant advantage of iPSCs is their potential for personalised cell therapy. Since these cells are derived from the patient’s own tissues, they are much less likely to be rejected by the immune system. This eliminates a major hurdle in traditional cell-based therapies, where the risk of immune rejection can be significant.
- The Future of Regenerative Medicine: Personalised cell therapies using patient-derived iPSCs hold immense promise for treating a wide range of diseases. These therapies could potentially involve transplanting healthy, lab-grown cells derived from a patient’s iPSCs to replace damaged or diseased cells. This approach offers the potential to treat conditions such as Parkinson’s disease, heart failure, and certain genetic disorders.
Distinguishing Factors Between MSCs and iPSCs:
The table below summarises the key differences between MSCs and iPSCs:
| Feature | MSCs | iPSCs |
| Origin | Adult tissues (bone marrow, fat, etc.) | Reprogrammed adult cells |
| Morphology | Elongated and spindle-shaped; Fibroblastic-like | More rounded morphology; Embryonic stem cell-like |
| Phenotype | CD73+, CD90+, CD105+, CD14−, CD19−, CD34−, CD45− | OCT4+, NANOG+, SOX2+, SSEA1+, SSEA3+, SSEA4+, TRA1-60+, TRA1-81+, ALP+ |
| Differentiation Potential | Multipotent (limited cell type) | Pluripotent (can become any cell type) |
| Clinical Applications | Tissue repair, immunomodulation | Disease modelling, personalised therapies |
By understanding these key distinctions between MSCs and iPSCs, we can appreciate the unique advantages each cell type brings to the field of regenerative medicine. MSCs offer readily available and potentially safer options for tissue repair and immunomodulation, while iPSCs hold the transformative potential for personalised medicine through disease modelling and cell-based therapies.
The Future of Stem Cell Therapies:
MSCs and iPSCs are not competing forces, but rather complementary tools with distinct strengths. As research progresses, we can expect to see these cell types play increasingly crucial roles in regenerative medicine. By harnessing their full potential, scientists can develop innovative therapies and potentially cure a wide range of diseases.
References:
Csobonyeiova M, Polak S, Nicodemou A, Zamborsky R, Danisovic L. iPSCs in Modeling and Therapy of Osteoarthritis. Biomedicines. 2021;9(2):186. Published 2021 Feb 12. doi:10.3390/biomedicines9020186
Rowe RG, Daley GQ. Induced pluripotent stem cells in disease modelling and drug discovery. Nat Rev Genet. 2019 Jul;20(7):377-388. doi: 10.1038/s41576-019-0100-z.
Song N, Scholtemeijer M, Shah K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol Sci. 2020;41(9):653-664. doi:10.1016/j.tips.2020.06.009
Thanaskody K, Jusop AS, Tye GJ, Wan Kamarul Zaman WS, Dass SA, Nordin F. MSCs vs. iPSCs: Potential in therapeutic applications. Front Cell Dev Biol. 2022 Nov 2;10:1005926. doi: 10.3389/fcell.2022.1005926.
Zomer HD, Vidane AS, Gonçalves NN, Ambrósio CE. Mesenchymal and induced pluripotent stem cells: general insights and clinical perspectives. Stem Cells Cloning. 2015 Sep 28;8:125-34. doi: 10.2147/SCCAA.S88036.
CONTACT

QUESTIONS IN YOUR MIND?
Connect With Our Technical Specialist.

KNOW WHAT YOU WANT?
Request For A Quotation.
OTHER BLOGS YOU MIGHT LIKE
HOW CAN WE HELP YOU? Our specialists are to help you find the best product for your application. We will be happy to help you find the right product for the job.

TALK TO A SPECIALIST
Contact our Customer Care, Sales & Scientific Assistance

EMAIL US
Consult and asked questions about our products & services

DOCUMENTATION
Documentation of Technical & Safety Data Sheet, Guides and more...
