- Your cart is empty
- Continue Shopping

How does Transdermal Patches Actually Deliver Drug To Our Body?
Learn how transdermal patches deliver medication through the skin, offering controlled, non-invasive drug delivery for medical and cosmetic use.


Cancer is a disease that can develop anywhere in our body as a result of excessive cell division. The location and cell types influence the etiology, symptoms, severity, and therapeutic approaches. In brain tumours, more than 75% of adults are affected by glioblastoma. The average survival rate is only two years after diagnosis, and the progression of therapeutic strategies is limited and challenging. These are mainly due to the blood-brain barrier (BBB) blockage, which makes intravenous chemotherapeutic compounds difficult to get through, and the relative rarity of this kind of cancer, which makes it difficult to attract pharmaceutical companies.
Currently, surgery, radiation, and chemotherapy are among the therapies utilized as a first line of treatment in the fight against brain cancer. However, these therapies frequently result in the patient’s health deteriorating, tumours recurring or spreading to other organs. These and other factors drive researchers to enhance brain tumour therapy approaches.
The use of mesenchymal stem cells (MSCs) as a delivery system is a promising treatment for brain cancer. Although the BBB prevents white blood cells from passing through it, it becomes compromised during inflammation, allowing those cells to be trafficked and directed to the inflamed site. Similarly, MSCs administered intravenously can pass through the BBB and target the injured site from their homing property. Researchers became interested in and used MSCs as a drug delivery method due to their non-invasive route of administration and homing properties.
MSC-based drug delivery systems can employ a number of different tactics (see figure). For instance, TNF-related apoptosis-inducing ligand (TRAIL)-engineered MSCs can be used to directly trigger apoptosis in cancer cells; MSCs primed with chemotherapy agents demonstrate anti-tumour and anti-angiogenic action; MSCs-expressing immunomodulatory substances like IL-2 and IFN-β can slow down tumour growth; adenovirus and measles virus are oncolytic viruses that preferentially reproduce in tumour cells, increasing the anti-tumour impact through re-infection phases; and tissue- or tumour-specific prodrugs that use certain enzyme-engineered MSCs to transform inactive substances into substances that can destroy tumours.
In conclusion, tremendous research efforts have produced a variety of cutting-edge treatment approaches. However, safety assurances, non-targeted cells’ homing issues, large population studies, and long-term follow-up are also significant aspects that enable researchers to investigate and develop MSCs-drug delivery treatments that secure the remission and well-being of patients.
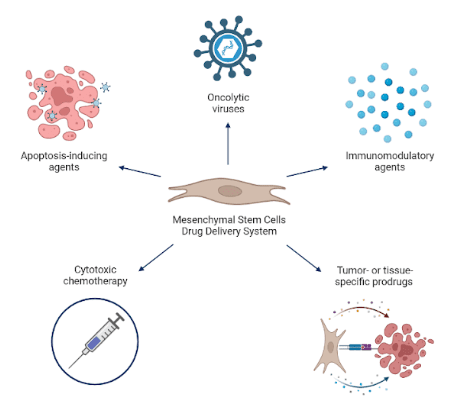
Mesenchymal stem cell-based drug delivery strategies
References

Connect With Our Technical Specialist.

Request For A Quotation.

Learn how transdermal patches deliver medication through the skin, offering controlled, non-invasive drug delivery for medical and cosmetic use.
Discover how AAV, LNPs, and exosomes advance neurological gene therapy, with imaging tools driving precision in CNS-targeted therapeutics.

Discover why hypothermic cell transport is safer and smarter than cryopreservation or room temp methods for preserving cell viability in transit.

Contact our Customer Care, Sales & Scientific Assistance

Consult and asked questions about our products & services

Documentation of Technical & Safety Data Sheet, Guides and more...