- Your cart is empty
- Continue Shopping
Make Your Single Cell Isolation Protocol Easy with CellGem

Make Your Single Cell Isolation Protocol Easy with CellGem
- Atlantis Bioscience
- Blog
- Reading Time: 7 minutes
Table of Contents
- Challenges of Single-Cell Isolation
- Common Methods for Single-Cell Isolation and Their Limitations
- What is CellGem and how does it help simplify single cell isolation protocols?
- How does CellGem address the limitations of current single cell isolation protocols?
- What are the applications of CellGem developed by Origem?
- 5 Tips for Single-Cell Isolation using CellGem
Isolating single cells is essential for various applications, including cell line development, monoclonal antibody production, single-cell multiomics, and the characterisation of cancer stem cells. To fully harness the potential of these applications, single-cell isolation must be fast, gentle, and efficient. In this blog, we will explore using the CellGem microfluidic chip to isolate single cells, highlighting its advantages and providing practical tips for achieving viable single-cell preparations.
Challenges of Single-Cell Isolation
Researchers face several challenges when isolating single cells. Achieving a pure population of the target cell type is crucial, but maintaining the integrity of cellular characteristics is equally important. Therefore, throughput (the number of cells isolated within a given time) and recovery (the ratio of isolated target cells to the total number of target cells in the original sample) are key factors to consider when selecting an appropriate technique.

Common Methods for Single-Cell Isolation and Their Limitations
Fluorescence-activated cell sorting (FACS) and limiting dilution are two widely utilised methods for single-cell isolation. FACS is known for its speed and high throughput, making it a popular choice for researchers. However, these advantages come with significant drawbacks. The combination of high pressure, electric charge, and the rapid succession of high-speed collisions during conventional FACS can induce cellular stress. This stress can negatively impact the viability and functionality of sorted cells, creating challenges for downstream applications, particularly with larger or more sensitive cell types, such as spheroids or neurons.
Limiting dilution, on the other hand, is a simpler and more accessible method that involves serially diluting a cell suspension to achieve single-cell isolation. While this technique is cost-effective and does not require specialised equipment, it also has its limitations. The efficiency of limiting dilution can be quite low, as it relies on the random distribution of cells in wells, which can result in a significant number of wells with no cells at all. Additionally, maintaining optimal culture conditions during the process is crucial; otherwise, the isolated cells may suffer from low viability.
Both methods also face logistical challenges. FACS typically requires a larger sample input, limiting its use for precious or scarce samples, such as clinical specimens. Furthermore, FACS instruments are often large and require specialised training, leading to accessibility issues. Similarly, while limiting dilution is more straightforward, it can be labour-intensive and time-consuming, necessitating careful planning and execution.
Given these limitations, researchers are increasingly exploring alternative methods for single-cell isolation that provide greater efficiency, flexibility, and viability. That’s where we found CellGem. A cutting-edge method for isolating single cells with high precision and efficiency using microfluidics principles.
Table 1: Comparison of CellGem, Limiting Dilution, and FACS
| Feature | CellGem | Limiting Dilution | FACS |
| Principle | Gravity-based microwell capture for single-cell isolation. | Serial dilution for random single-cell distribution. | Electric charge and fluorescence-based sorting. |
| Throughput | High, processes many cells efficiently. | Low, slow process. | High, sorts thousands of cells per second. |
| Cell Viability & Stress | Gentle, preserves viability of sensitive cells. | Low stress but inefficient. | High stress, reduces viability of sensitive cells. |
| Sample Input | Works with small, rare samples. | Minimal input, but low efficiency. | Requires large sample input. |
| Equipment | Simple, user-friendly device. | Basic equipment, labour-intensive. | Expensive, specialised equipment needed. |
| Efficiency | High-precision capture. | Low, random capture efficiency. | High efficiency, but stress can affect outcomes. |
| Cell Type | Suitable for various, including sensitive cells. | Limited by culture conditions. | Broad, but large or fragile cells may suffer. |
| Cost | Moderate, no specialised infrastructure. | Low cost, but time-consuming. | High cost for equipment and maintenance. |
| Time Requirement | Fast, with high capture efficiency. | Slow and laborious. | Fast, but requires preparation and setup. |
What is CellGem and how does it help simplify single cell isolation protocols?
The Principle of CellGem
CellGem is a microfluidic chip featuring arrays of microwells on each side, specifically designed for capturing and culturing single cells. The operation of the device involves several key steps:
- Cell Suspension Loading: A prepared cell suspension is loaded into the CellGem device, allowing cells to settle into the capture wells by gravity.
- Cell Capture: Each capture well is designed to trap a single cell, ensuring that individual cells are isolated from the rest of the suspension.
- Transfer to Culture Wells: After the cells are captured, the device is flipped, allowing the isolated cells to fall from the capture wells into adjacent culture wells, again utilising gravity for this transfer.
- Cell Growth and Division: Once in the culture wells, each cell begins to grow and divide, forming a single-cell colony. These colonies can then be easily transferred to other culture vessels for further expansion and experimentation.
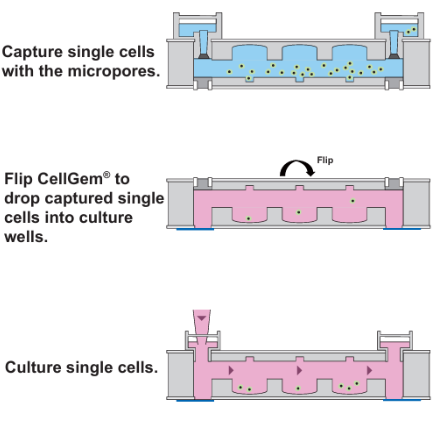
Figure 1: Principle of single cell isolation and culture using CellGem.
How does CellGem address the limitations of current single cell isolation protocols?
- Gentle Isolation Process: CellGem employs a gentle isolation technique that minimises cellular stress, ensuring high viability and functionality of the isolated cells. This is particularly important for sensitive cell types, such as stem cells and neurons, which can be adversely affected by harsh sorting conditions.
- High Throughput: The technology is designed to process large numbers of cells rapidly, making it suitable for applications requiring high throughput. This efficiency allows researchers to isolate more cells in less time, streamlining workflows.
- Low Sample Input Requirement: Unlike traditional FACS methods that require substantial sample volumes, CellGem can work effectively with smaller samples, making it ideal for precious clinical specimens or rare cell types.
- Scalability: CellGem is scalable, meaning it can adapt to various experimental needs, from small-scale studies to larger projects. This flexibility is crucial for researchers who may need to increase sample sizes or expand their experiments over time.
- User-Friendly Operation: The CellGem system is designed to be easy to use, requiring less specialised training compared to traditional sorting technologies. This accessibility allows a broader range of researchers to utilise the method effectively.
- Preservation of Cell Characteristics: By maintaining the physiological properties of cells during the isolation process, CellGem ensures that the sorted cells retain their characteristics, which is essential for downstream applications like functional assays and genomic studies.
- Integration with Downstream Applications: The technology is compatible with various downstream applications, including single-cell RNA sequencing, functional assays, and cellular therapies, making it a versatile tool for modern cell biology.
What are the applications of CellGem developed by Origem?
CellGem is a versatile tool that offers a wide range of applications in research and biotechnology. Its capacity for efficient single-cell isolation and culture is particularly valuable in several key areas:
- Cell Line Development: CellGem enables the isolation of single cells for the creation of homogeneous cell lines, essential for drug development, therapeutic testing, and basic research. In addition to generating consistent cell populations, CellGem can be integrated with gene editing technologies like CRISPR-Cas9. By isolating gene-edited cells at the single-cell level, researchers can select clones with precise genetic modifications. This ensures accuracy in studies that require specific gene alterations, such as disease modelling, functional genomics, or the production of therapeutic proteins.
- Single-Cell Multiomics: The technology facilitates the analysis of multiple omic layers—genomics, transcriptomics, and proteomics—from individual cells. This comprehensive approach provides deep insights into cellular functions, interactions, and heterogeneity. By examining these layers, researchers can uncover intricate details about cellular pathways, gene expression, and protein profiles, enhancing our understanding of complex biological systems.
- Applications in Cancer Research: CellGem plays a crucial role in cancer research by enabling the study of cancer stem cells (CSCs). CSCs are a small, distinct population of cells within tumours that possess self-renewal capabilities and drive tumour growth. By isolating single CSCs, researchers can investigate their unique characteristics, such as differentiation pathways, treatment resistance, and contributions to tumour heterogeneity. This knowledge is vital for developing targeted therapies and improving treatment outcomes in cancer patients.
- Monoclonal Antibody Production: CellGem supports the production of monoclonal antibodies by facilitating the isolation of hybridoma cells. These hybridomas are formed by fusing B cells, which produce specific antibodies, with myeloma cells that can proliferate indefinitely. By isolating single hybridoma cells, researchers can generate stable populations that consistently produce the desired antibody, which is critical for targeted therapies and diagnostic applications.

5 Tips for Single-Cell Isolation using CellGem
Achieving efficient and consistent single-cell isolation and cloning requires careful attention to various factors that can significantly impact outcomes. If you’re planning to simplify your single cell isolation protocol with CellGem, here are 5 important tips and consideration to help you optimise your success in these processes:
1. Optimal Cell Condition and Density
For successful single-cell isolation, use cells in the logarithmic (log) phase of growth to ensure high viability and responsiveness. The right cell density is crucial—target a concentration of around 1 x 10^6 cells/mL, but be prepared to experiment with different densities to find the optimal conditions for your specific cell type.
2. Cell Health and Viability
Healthy cells are vital for successful isolation. Make sure the cells are free from contamination, and assess their morphology regularly to confirm optimal health. Healthier cells are more likely to grow successfully after isolation, so maintaining ideal culture conditions is essential.
3. Gentle Handling Techniques
Handle cells with care during isolation to minimise stress. Be mindful of pipetting forces, as excessive agitation can compromise cell integrity. During cell loading, avoid disrupting the cells or creating air bubbles, which can hinder capture efficiency. Handle the CellGem chip with caution to prevent bubble formation, as air bubbles can interfere with the chip’s performance.
4. Single-Cell Suspension Preparation
Speed is key when preparing a single-cell suspension—minimise preparation time to maintain cell viability. Filter out clumps using a cell strainer, and ensure cells are fully resuspended before injecting them into the CellGem chip.
5. Optimising Cell Capture Efficiency in CellGem
Follow these best practices to maximise the capture efficiency of single cells with CellGem:
- Allow Cells to Settle: After seeding the cell suspension, allow at least 3 minutes for the cells to settle into the capture wells. Rushing this step may reduce capture efficiency.
- Select the Correct Capture Well Size: Ensure the capture well size is appropriate for your cell type. Measure the diameter of your cells beforehand and select the corresponding well size for optimal capture.
- Multiple Cell Loading: For better capture efficiency, load cells into the chip multiple times. This increases the chance of capturing single cells if the initial load is insufficient.
- Wash Wells Thoroughly: After seeding, wash the wells at least twice to remove untrapped or excess cells. This ensures that only captured cells remain in the wells for optimal growth and reduces overcrowding.
- Microscope Verification: After loading the cells, verify under a microscope to ensure successful capture. If cells are not properly captured, reload the suspension and flush again to enhance trapping.
References:
Gross, A.; Schoendube, J.; Zimmermann, S.; Steeb, M.; Zengerle, R.; Koltay, P. Technologies for Single-Cell Isolation. Int. J. Mol. Sci. 2015, 16, 16897-16919. https://doi.org/10.3390/ijms160816897
Hu P, Zhang W, Xin H and Deng G (2016) Single Cell Isolation and Analysis. Front. Cell Dev. Biol. 4:116. doi: 10.3389/fcell.2016.00116
Yeh, C.-F.; Lin, C.-H.; Chang, H.-C.; Tang, C.-Y.; Lai, P.-T.; Hsu, C.-H. A Microfluidic Single-Cell Cloning (SCC) Device for the Generation of Monoclonal Cells. Cells 2020, 9, 1482. https://doi.org/10.3390/cells9061482
CONTACT

QUESTIONS IN YOUR MIND?
Connect With Our Technical Specialist.

KNOW WHAT YOU WANT?
Request For A Quotaiton
OTHER BLOGS YOU MIGHT LIKE
HOW CAN WE HELP YOU? Our specialists are to help you find the best product for your application. We will be happy to help you find the right product for the job.

TALK TO A SPECIALIST
Contact our Customer Care, Sales & Scientific Assistance

EMAIL US
Consult and asked questions about our products & services

DOCUMENTATION
Documentation of Technical & Safety Data Sheet, Guides and more..

