- Your cart is empty
- Continue Shopping
A Pioneering Leap in Regenerative Medicine: iMatrix 511 and the Future of Corneal Transplants

A Pioneering Leap in Regenerative Medicine: iMatrix 511 and the Future of Corneal Transplants
- Atlantis Bioscience
- Blog
- Reading Time: 5 minutes
While significant advancements have been made in the field of regenerative medicine in recent years, a 2019 breakthrough paved the way for a future filled with even greater possibilities. This milestone involved a successful corneal transplant using induced pluripotent stem cells (iPSCs) – and a key player in this achievement was Matrixome’s innovative product, iMatrix 511.
The Challenge: A Scarce Resource
Cornea transplants are a life-changing procedure for individuals suffering from corneal diseases or injuries. They are, in fact, the most commonly performed transplant surgery worldwide. However, despite this high demand, there’s a critical bottleneck in the system: the availability of donor corneas.
The number of people needing corneal transplants far outpaces the supply of donor tissue, resulting in lengthy waiting times. Donated corneas can vary in quality due to factors like the age of the donor, cause of death, and time between death and tissue retrieval. In some cases, rejection of the donor cornea by the recipient’s immune system can occur. These limitations of donor corneas highlight the urgent need for alternative solutions in corneal transplantation.
A Groundbreaking Solution: iMatrix and the Future of Corneal Transplants
iPSC technology offers a revolutionary solution for corneal transplants. By reprogramming a patient’s own cells or those of a matched donor into a pluripotent state, scientists can create a virtually limitless source of corneal cells. This eliminates the dependence on scarce donor corneas and significantly reduces the risk of rejection.
In 2019, a team of researchers led by ophthalmologist Kohji Nishida at Osaka University in Japan made history by performing the world’s first successful corneal transplant using iPSC technology. As part of a clinical trial, the team performed a cornea transplant on a woman in her 40s with a corneal disease that caused her to lose these stem cells. The iPSCs used in the transplant were taken from an adult donor, reprogrammed into an undifferentiated state, and then induced to develop into corneal stem cells. Corneal epithelial cell sheets were generated and successfully transplanted into the patient. Three other patients were also subsequently transplanted with allogeneic iPSCs-derived corneal epithelial cell sheets. Encouragingly, none of the patients developed complications like rejection or tumor growth from the transplanted cells. Furthermore, all patients reported improvement in their symptoms within a few years of the transplant.
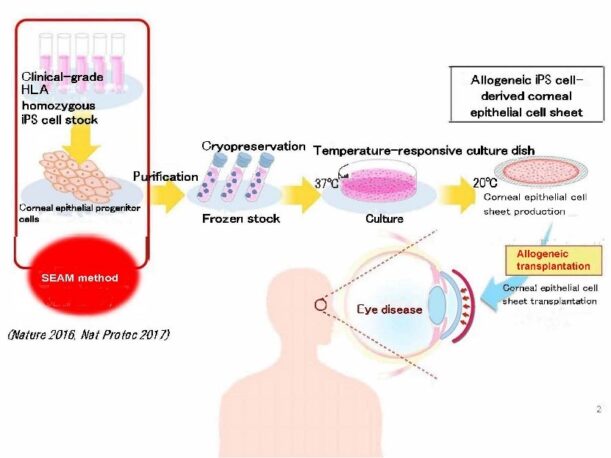
Figure 1: Workflow of allogenic transplantation of iPSC-derived corneal epithelial cell sheet.
This clinical study examines the safety (major) and efficacy of corneal epithelial tissue by inducing corneal epithelial cells using iPSCs using a uniquely developed method, and then culturing them into sheets.
Here’s where iMatrix 511 comes in. This biomaterial provides a critical scaffold to support the growth and organisation of these iPSCs-derived corneal cells. iMatrix 511 mimics the natural extracellular matrix (ECM) – the complex network that provides structural and biochemical cues for cell function – thereby promoting the development of healthy corneal tissue.
The Road Ahead: Building on a Promising Future
The successful use of iPSC-derived corneal epithelial cells in 2019 marked a major breakthrough in eye disease treatment. In fact, there have been several studies exploring the potential of other iPSC-derived cells for various eye conditions. These include iPSC-derived corneal endothelial cells for diseases affecting the cornea’s inner layer and iPSC-derived retinal pigmented epithelium (RPE) cells for age-related macular degeneration (AMD), a leading cause of vision loss. Notably, successes have been achieved, and clinical trials for iPSC-derived RPE transplantation in AMD patients are already underway.
While these advancements represent significant progress, further research and development are necessary to refine iPSC corneal and retinal transplant techniques before they can be widely applied in clinical settings. This involves optimising iPSC generation methods, ensuring the consistency and quality of derived corneal tissue, and developing minimally invasive surgical techniques for transplantation.
Despite the challenges ahead, the potential of iPSC therapy, supported by biomaterials like iMatrix 511, is undeniable. Continued advancements could revolutionise both corneal and retinal transplantation, providing a readily available source of high-quality, personalised tissue for patients in need. This could significantly reduce wait times, improve transplant success rates, and ultimately restore sight, enhancing the lives of millions worldwide.
iMatrix: At the Forefront of Regenerative Medicine
The story of iMatrix 511 begins with over a decade of groundbreaking research by Dr. Kiyotoshi Sekiguchi at Osaka University. This research focused on the extracellular matrix (ECM), the complex network that provides structural and biochemical cues for cell function. Inspired by this work, Matrixome developed the iMatrix™ product line, with iMatrix-511 as its cornerstone.
iMatrix-511 is a highly purified and refined fragment of laminin-511 E8, a key component of the ECM. It retains the crucial integrin binding activity of full-length laminin-511, acting as a biomimetic scaffold that closely resembles the natural cellular environment. This supportive structure plays a vital role in promoting the efficient growth and differentiation of various cell types, including iPSCs.
The beauty of iMatrix-511 lies in its versatility. Researchers can seamlessly transition between RUO (Research Use Only) iMatrix-511 for basic research and iMatrix-511MG, a clinically-grade version, for potential use in clinical trials and applications. This streamlined approach facilitates the translation of scientific discoveries into tangible therapeutic benefits.
While the use of iMatrix-511 in iPSC-based corneal transplants is a significant advancement, its applications extend far beyond this field. A noteworthy example of iMatrix-511MG’s potential is its use at the Kyoto University Center for iPS Cell Research & Application (CiRA) for their Clinical iPSC Stock Project. This project aims to generate clinical-grade iPSCs for various iPSC-based therapies. Some of these include those targeting spinal cord injury and Parkinson’s disease.
Looking Forward
The remarkable success of iMatrix-511 in generating clinical-grade iPSCs at CiRA highlights its immense potential as a cornerstone for future cell-based therapies. This biomaterial offers renewed hope for millions by facilitating the development of treatments for a wide range of debilitating conditions.
As the field of regenerative medicine continues its rapid evolution, iMatrix-511 is poised to play a pivotal role. Research will undoubtedly explore its potential further, potentially leading to the development of new biomaterials tailored to specific tissues or even combination therapies that leverage iMatrix-511 alongside other regenerative medicine techniques. The future of regenerative medicine in eye disease and other diseases is undeniably bright, and iMatrix 511 stands as a powerful tool for shaping this exciting landscape, ultimately offering the promise of improved lives for countless patients.
References:
Gain P, Jullienne R, He Z, et al. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016;134(2):167-173. doi:10.1001/jamaophthalmol.2015.4776
Hayashi R, Ishikawa Y, Katori R, et al. Coordinated generation of multiple ocular-like cell lineages and fabrication of functional corneal epithelial cell sheets from human iPS cells. Nat Protoc. 2017;12(4):683-696. doi:10.1038/nprot.2017.007
Hayashi R, Ishikawa Y, Sasamoto Y, et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature. 2016;531(7594):376-380. doi:10.1038/nature17000
Hatou S, Sayano T, Higa K, et al. Transplantation of iPSC-derived corneal endothelial substitutes in a monkey corneal edema model. Stem Cell Res. 2021;55:102497. doi:10.1016/j.scr.2021.102497
Mandai M, Watanabe A, Kurimoto Y, et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N Engl J Med. 2017;376(11):1038-1046. doi:10.1056/NEJMoa1608368
Shibata S, Hayashi R, Kudo Y, et al. Cell-Type-Specific Adhesiveness and Proliferation Propensity on Laminin Isoforms Enable Purification of iPSC-Derived Corneal Epithelium. Stem Cell Reports. 2020;14(4):663-676. doi:10.1016/j.stemcr.2020.02.008
Shibata S, Hayashi R, Okubo T, et al. Selective Laminin-Directed Differentiation of Human Induced Pluripotent Stem Cells into Distinct Ocular Lineages. Cell Rep. 2018;25(6):1668-1679.e5. doi:10.1016/j.celrep.2018.10.032
Sugai K, Sumida M, Shofuda T, Yamaguchi R, Tamura T, Kohzuki T, Abe T, Shibata R, Kamata Y, Ito S, Okubo T, Tsuji O, Nori S, Nagoshi N, Yamanaka S, Kawamata S, Kanemura Y, Nakamura M, Okano H. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Regen Ther. 2021 Sep 7;18:321-333. doi: 10.1016/j.reth.2021.08.005.
Takahashi J. iPS cell-based therapy for Parkinson’s disease: A Kyoto trial. Regen Ther. 2020;13:18-22. Published 2020 Sep 15. doi:10.1016/j.reth.2020.06.002
Umekage M, Sato Y, Takasu N. Overview: an iPS cell stock at CiRA. Inflamm Regen. 2019 Sep 2;39:17. doi: 10.1186/s41232-019-0106-0.
CONTACT

QUESTIONS IN YOUR MIND?
Connect With Our Technical Specialist.

KNOW WHAT YOU WANT?
Request For A Quotaiton
OTHER BLOGS YOU MIGHT LIKE
HOW CAN WE HELP YOU? Our specialists are to help you find the best product for your application. We will be happy to help you find the right product for the job.

TALK TO A SPECIALIST
Contact our Customer Care, Sales & Scientific Assistance

EMAIL US
Consult and asked questions about our products & services

DOCUMENTATION
Documentation of Technical & Safety Data Sheet, Guides and more..