- Your cart is empty
- Continue Shopping
A Guide From AAV Vector Design to Quality Control

A Guide From AAV Vector Design to Quality Control
- Atlantis Bioscience
- Blog
- Reading Time: 15 minutes
Adeno-associated viruses (AAVs) have become a cornerstone of gene therapy, offering a powerful platform for delivering therapeutic genes to both dividing and non-dividing cells. However, the success of AAV-based therapies hinges on their ability to target specific tissues, precise vector design, and rigorous quality control. Optimising vector design—through careful selection of serotype, promoter, and cargo—directly impacts transduction efficiency, tissue specificity, and transgene expression. At the same time, stringent quality control is essential to ensure AAV preparations’ safety, potency, and consistency, minimising risks such as off-target effects and immunogenicity.
Several AAV-based therapies have already secured regulatory approval for conditions such as hemophilia B, spinal muscular atrophy, and retinal dystrophy, underscoring the clinical relevance of well-engineered vectors and robust QC strategies. This guide will explore the critical factors influencing AAV vector design and the key quality control measures necessary for developing safe and effective gene therapies.
Designing Your AAV Vector – Key Considerations
The success of AAV-based gene therapy starts with strategic vector design, as this directly impacts transduction efficiency, gene expression, and overall therapeutic efficacy. Proper design ensures that the vector delivers the transgene efficiently, maintains stability, and avoids unwanted immune responses.
1. Selecting the Right AAV Serotype
AAVs have 13 naturally occurring serotypes (AAV1–AAV13) and numerous engineered variants. Each serotype differs in its capsid protein composition, influencing receptor binding, tissue tropism, and immune response. These differences make serotype selection a critical factor in gene therapy, as it directly impacts transduction efficiency and specificity for target tissues.
Natural AAV Serotypes and Their Tropism
The AAV capsid, formed by 60 monomers of VP1, VP2, and VP3 structural proteins, determines the virus’s interaction with host cells. While all AAV serotypes share a conserved icosahedral structure, variations in their capsid proteins influence receptor binding and tissue preference. For instance:
- AAV2 binds to heparan sulfate proteoglycan (HSPG), enabling broad tropism across the tissues.
- AAV9 interacts with galactose, facilitating efficient transduction of the central nervous system (CNS) and cardiac tissues.
- AAV1, AAV4, AAV5, and AAV6 bind to sialic acid proteoglycans, dictating their unique tissue-targeting properties.
Choosing the appropriate AAV serotype involves evaluating the target tissue, transduction efficiency, immune response, and off-target effects. Systematic screening, such as comparing serotypes using reporter gene expression via microscopy or flow cytometry, can guide selection for optimal gene delivery.
Table 1: AAV Tissue Tropism (Addgene)
| Liver | Heart | SK muscle | Photoreceptor Cells | Retinal Pigment Epithelium | CNS | Kidney | Pancreas | Lung | |
| AAV1 | √ | √ | √ | √ | |||||
| AAV2 | √ | √ | √ | ||||||
| AAV3 | |||||||||
| AAV4 | √ | √ | √ | ||||||
| AAV5 | √ | √ | √ | √ | |||||
| AAV6 | √ | √ | √ | ||||||
| AAV7 | √ | √ | |||||||
| AAV8 | √ | √ | √ | √ | √ | √ | √ | √ | |
| AAV9 | √ | √ | √ | √ | √ |
Why Engineer New Capsids?
While natural AAV serotypes offer diverse tropisms, they have limitations in efficiency, specificity, and immune evasion. This has inspired the generation of novel AAV capsid through capsid engineering to address these challenges:
- Enhancing transduction efficiency in specific cell types.
- Modifying tropism to target previously inaccessible tissues.
- Reducing immune recognition to prolong vector circulation and minimise host immune responses.
Through rational design, directed evolution, and machine learning-driven approaches, researchers continue to develop novel AAV variants with improved therapeutic potential. Engineered capsids are particularly promising for treating genetic disorders and advancing gene therapy applications in previously untreatable conditions.
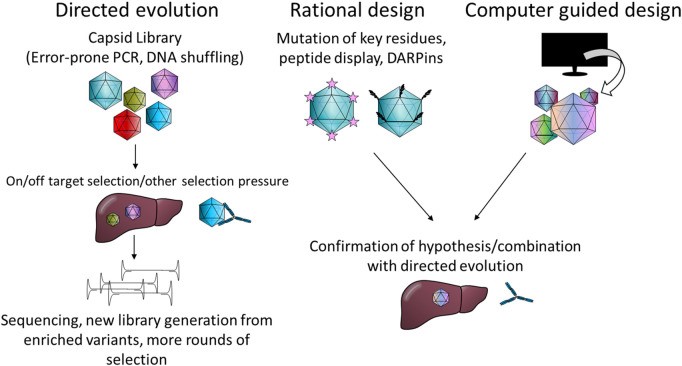
Reference: doi: 10.3389/fmmed.2022.1054069
PackGene’s AAV Capsid Engineering Platform – π-Icosa System
PackGene offers a cutting-edge π-Icosa system for engineering and screening AAV capsid variants with enhanced features, such as improved organ targeting, reduced off-target effects, and other customised functionalities. This platform involves a three-phase process to identify top-performing capsid variants for gene therapy development:
- Phase I:
- Construct an initial AAV capsid library.
- Package AAVs, inject into animals, and conduct initial testing.
- Perform NGS analysis and refine the library for Phase II if needed.
- Phase II:
- Use the refined library from Phase I to repeat packaging, animal injection, and testing.
- Identify top capsid variants through advanced screening.
- Phase III:
- Construct plasmids for the top variants, package AAVs, and conduct large-scale animal testing.
- Optional histology tests are available for deeper analysis
This systematic approach ensures the identification of highly efficient and specific AAV capsids tailored to your gene therapy needs.
2. Optimising the Transgene Cassette
The transgene cassette is a critical component of AAV vector design, directly influencing expression levels, duration, and safety. Careful selection of promoters, enhancers, and regulatory elements ensures precise and efficient gene delivery while minimising unintended effects.
A. Promoter Selection
The promoter is a critical component of AAV vector design, influencing the level, specificity, and timing of transgene expression. Promoters fall into three categories:
- Constitutive Promoters (e.g., CMV, CAG): Drive strong, continuous expression across many cell types, ideal for broad applications but may cause off-target effects.
- Tissue-Specific Promoters (e.g., GFAP for astrocytes, Albumin for liver, Synapsin for neurons): Restrict expression to specific tissues, enhancing precision and safety for targeted therapies.
- Inducible Promoters (e.g., tetracycline-, chemical-, or light-inducible systems): Enable controlled expression in response to external stimuli, useful for conditional gene regulation.
Balance expression strength, specificity, and control. Constitutive promoters are best for broad expression, tissue-specific promoters for targeted delivery, and inducible systems for precise regulation. The choice depends on therapeutic goals, target tissue, and desired control over transgene expression.
B. Transgene Payload Size Considerations
When designing AAV vectors for gene therapy, the size of the transgene payload is a critical factor. AAVs are engineered in two primary forms: single-stranded AAV (ssAAV) and self-complementary AAV (scAAV), each with distinct packaging capacities and mechanisms of transgene expression.
ssAAV has a packaging limit of ~5 kb, including the inverted terminal repeats (ITRs) that flank the transgene. Exceeding this limit reduces packaging efficiency, and inserts larger than 4.7 kb may only be partially packaged.
scAAV, while offering faster transgene expression due to its self-complementary double-stranded DNA structure, has a more limited capacity of ~2.5 kb. Although this can be extended to 3.3 kb, the proportion of single-stranded genomes increases with size, reducing efficiency.
For larger transgenes (>5 kb), innovative approaches such as fragmented packaging, overlapping dual vectors, and trans-splicing dual vectors are utilised. These methods rely on homologous recombination, overlapping sequences, or ITR concatemerization to reconstruct the full gene in target cells. Hybrid strategies combining overlapping and trans-splicing techniques further enhance efficiency by addressing potential issues with incomplete gene reconstruction or unwanted ITR structures.
Careful consideration of transgene payload size is essential for optimising AAV vector design. Staying within packaging limits or employing advanced strategies for oversized genes ensures efficient delivery, robust transgene expression, and successful therapeutic outcomes.
C. Optimising Protein Labelling
Protein labelling enables the detection, tracking, and analysis of proteins in biological systems. When designing AAV vectors, integrating labelling elements (tags, fluorescent proteins, linkers) requires careful planning to balance functionality with AAV’s limited packaging capacity (~4.7–5.0 kb for ssAAV, ~2.5–3.3 kb for scAAV).
Strategies for Protein Labelling
- Small Tags (e.g., Flag, HA, 6His): Used for protein purification, immunoprecipitation, and Western blotting. Compact and minimally disruptive, they are ideal for AAV vectors.
- Fluorescent Proteins (e.g., GFP, mCherry): Enable live-cell imaging and FACS sorting. Can be fused directly to the transgene or separated by self-cleaving peptides (e.g., T2A, P2A) to conserve space.
- Size Optimisation: Large tags reduce packaging efficiency. Use smaller tags or dual-vector systems for oversized constructs.
Design Considerations
- Tag Placement: N-terminal or C-terminal, depending on protein function.
- Linkers: Flexible linkers maintain protein stability and functionality.
- Multifunctional Tags: Combine small tags with fluorescent proteins for dual-purpose applications.
- Alternative Strategies: Use split-protein systems or dual vectors for complex labelling needs.
Applications
- Live-Cell Imaging: Track protein dynamics in real time.
- Protein Interactions: Study interactions using proximity ligation assays.
- Therapeutic Monitoring: Monitor expression and localisation of therapeutic proteins in vivo.
- Cell Sorting: Isolate specific cell populations using FACS.
Protein labelling is essential for studying protein function, but its integration into AAV vectors requires careful optimisation of tag size, placement, and design. By employing strategies like self-cleaving peptides or dual vectors, researchers can achieve effective labelling without compromising AAV packaging efficiency, advancing both research and therapeutic applications.
D. Polyadenylation Signals and Regulatory Elements
AAV vector design requires careful integration of regulatory elements to ensure efficient transgene expression. Key components include:
Polyadenylation Signals
- Function: Direct the addition of a poly(A) tail to mRNA, stabilising transcripts and enhancing translation.
- Common Signals:
- SV40 polyA: Strong and reliable.
- BGH polyA: Efficient mRNA stabilisation.
- Synthetic polyA (e.g., miniPolyA): Compact and efficient, ideal for AAV’s limited packaging capacity.
Regulatory Elements
- Introns (e.g., chimeric intron, SV40 intron): Improve mRNA stability, nuclear export, and translation efficiency.
- Enhancers (e.g., CMV enhancer, tissue-specific enhancers): Boost transcriptional activity of promoters.
- UTRs:
- 5′ UTR: Enhances translation initiation (e.g., Kozak sequence).
- 3′ UTR: Regulates mRNA stability and degradation.
- WPRE: Increases mRNA stability and nuclear export, enhancing transgene expression.
Design Considerations
- Packaging Capacity: Use compact elements (e.g., synthetic polyA, minimal introns) to conserve space.
- Tissue-Specific Regulation: Incorporate tissue-specific enhancers or UTRs for targeted expression.
- Expression Stability: Combine strong polyA signals with WPRE for robust, long-lasting expression.
Polyadenylation signals and regulatory elements are essential for optimising mRNA processing, stability, and translation in AAV vectors. By carefully selecting and balancing these elements, researchers can maximise transgene expression within AAV’s packaging limits, ensuring effective and safe gene therapies.
3. Immune Response and Safety
While AAV vectors have low immunogenicity, immune responses can still affect transduction efficiency, vector performance, and patient safety. Managing these responses is crucial for successful gene therapy.
Mitigating CpG-Triggered Immune Activation
High CpG content in transgene sequences can activate innate immunity, causing inflammation and reduced expression. Strategies to minimise this include:
- Codon Optimisation – Redesigning transgene sequences to reduce CpG motifs while maintaining expression.
- CpG Depletion – Removing CpG dinucleotides from plasmid backbones and transgene cassettes to lower immune activation.
Reducing Immunogenicity
- Avoiding Strong Viral Promoters – Viral promoters like CMV can trigger immune responses; tissue-specific or synthetic promoters help reduce this risk.
- Tissue-Specific Expression – Restricting expression to target tissues minimises off-target effects and immune activation.
- Empty Capsid Removal – Purifying AAV preparations to eliminate empty capsids, which can provoke immune responses without delivering therapeutic payloads.
Addressing immunogenicity is key to optimising AAV-based gene therapies. By incorporating strategies like CpG reduction, tissue-specific expression, and immune modulation, researchers can enhance vector performance and improve patient safety, advancing AAV vectors toward safe and effective clinical use.
Selecting a Production System
AAV vector production relies on specialised packaging systems, each with distinct advantages and limitations. The choice of production system impacts yield, scalability, consistency, and regulatory compliance, making it a critical consideration for gene therapy applications. Below are some commonly used AAV production systems.
Triple Transfection in HEK 293 Cells
Transfection-based production methods remain the most commonly used approach for laboratory-scale AAV vector production. This method relies on the transient transfection of HEK293 cells with three plasmids: one encoding the rAAV vector construct, another supplying the rep and cap genes, and a third containing adenoviral helper genes (E2A, E4orf6, and VA RNA). HEK293 cells provide the essential E1A and E1B functions necessary for AAV production. A simplified alternative employs double transfection using two plasmids, where the rep/cap and adenoviral helper functions are combined into a single plasmid. Despite its flexibility in allowing rapid serotype and transgene modifications, transfection-based production poses challenges in scalability, as it requires significant amounts of plasmid DNA, increasing production costs. Furthermore, variability in transfection efficiency affects batch-to-batch reproducibility, complicating clinical applications.
- Baculovirus-Based Production System
The baculovirus-based production system involves infecting suspension Sf9 insect cells with baculoviruses encoding the rep, cap, and rAAV vector construct. While this method enables large-scale production, early iterations suffered from genetic instability in the rep gene and insufficient VP1 expression, reducing vector yield and infectivity. Subsequent optimisations, including reducing the number of required baculoviruses and consolidating genetic elements into a single construct (Monobac system), have improved stability and efficiency. The use of a lower multiplicity of infection (MOI) further enhanced co-infection rates, resulting in increased AAV vector yield.
Despite its advantages, baculovirus-based production system faces challenges, mainly due to baculovirus genetic instability, which leads to defective interfering particles that can outcompete functional viruses and reduce rAAV yields. Stability improvements include targeted gene deletions and transgene insertions into stable loci. Baculovirus-based production system also struggles to assemble AAV capsids in the optimal VP1:VP2:VP3 ratio (1:1:10), lowering transduction efficiency. Further research is needed to optimise baculovirus-based production system for rAAV production.
- Stable Mammalian Cell Lines
Stable mammalian cell lines offer scalability, reproducibility, and cost-effectiveness for rAAV production. There are two main types: packaging and producer cell lines. Packaging cell lines stably express AAV Rep/Cap genes but require infection with wild-type adenovirus (Ad) to initiate expression. Following this, a secondary infection with an Ad-AAV hybrid virus allows the rescue, replication, and packaging of rAAV genomes. In contrast, producer cell lines have Rep/Cap, Ad helper genes, and the rAAV genome stably integrated into the host genome. In these cells, infection with wild-type Ad activates Rep/Cap expression, facilitating rAAV production without the need for a secondary infection.
Several HeLa-based stable cell lines, such as C12, H44, and B50, have demonstrated high yields of infectious and replication-competent AAV (rcAAV)-free rAAV through endogenous Rep/Cap expression. Additionally, an A549-derived stable cell line expressing Rep/Cap and a K209 cell line have been reported to produce high yields of infectious rAAV per cell. Stable cell lines derived from A549 and HEK293 cells have also been adapted for suspension cultures, making them suitable for scalable rAAV production.
Despite their advantages, stable cell lines and Ad infection-based rAAV production systems come with challenges. Developing stable cell lines is a complex and time-consuming process that is specific to the serotype and vector genome. Characterising and ensuring the stability of these cell lines can also be difficult, as passage history may impact growth kinetics. Additionally, to ensure the safety of the final gene therapy product, robust downstream purification processes are essential to remove any pathogenic Ad contaminants and oncogenic HeLa DNA.
Table 2: Comparison of the Common AAV Production Systems
| Production System | Advantages | Limitations |
| Triple Transfection (HEK293) | Versatility/flexibility Helper virus-free | Low scalability and high cost |
| Baculovirus/Insect Cells | High yield and scalability | Genetic instability of the baculovirus Optimisation needed for proper VP1/2/3 ratio |
| Stable Mammalian Cell Lines | High yield and scalability | Process to establish stable cell lines Limited flexibility Risk if Ad contamination |
Ensuring AAV Quality – Critical QC Measures
Ensuring the quality of AAV vectors is critical for their safety, efficacy, and consistency in gene therapy applications. AAV vector manufacturing platforms must incorporate ongoing characterisation of both process intermediates and final products. This continuous monitoring enables process optimisation while ensuring compliance with regulatory standards.
Key quality attributes of AAV vectors include purity, genomic integrity, potency, stability, and sterility. These factors influence transduction efficiency, biodistribution, and immunogenicity, making their assessment essential for therapeutic success. Routine characterisation of these attributes provides valuable insights into product consistency and safety.
The following sections outline critical QC measures for AAV vectors and the analytical techniques commonly used to evaluate them.
1. Purity and Impurity Analysis
Ensuring the purity of AAV vectors is crucial for their efficacy and safety. One of the primary challenges in AAV manufacturing is the presence of product-related impurities, including empty and partially filled capsids. These impurities can compete with full capsids for cellular entry, reducing transduction efficiency while also increasing the risk of immune responses due to higher viral loads.
Capsid Ratio Analysis
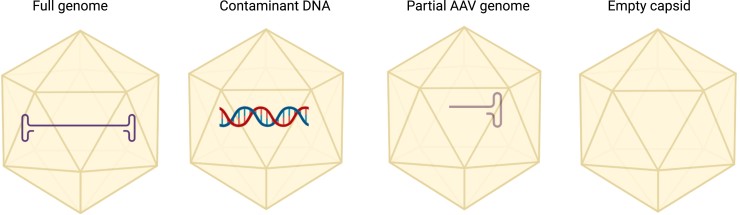
A critical quality control parameter in AAV production is the full-to-empty capsid ratio. Regulatory standards typically aim for a high proportion of full capsids (≥70%) to maximise potency while minimising unnecessary viral load. Several analytical techniques are employed to assess this ratio:
- Capillary isoelectric focusing (cIEF): Separates AAV capsids based on charge differences, enabling quantification of full and empty particles.
- Ion exchange chromatography (IEC/AEX): Distinguishes capsids based on charge properties, commonly used in process optimisation.
- Analytical ultracentrifugation (AUC): Provides high-resolution separation of full, partial, and empty capsids based on sedimentation rates.
- Transmission electron microscopy (TEM): Directly visualises capsid populations, offering qualitative and semi-quantitative insights.
- Charge detection mass spectrometry (CDMS): Measures capsid charge-to-mass ratio, allowing accurate determination of genome packaging.
- Size-exclusion chromatography coupled with multi-angle light scattering (SEC-MALS): Assesses capsid size and heterogeneity, providing additional insights into purity.
Optimising Capsid Purity
To achieve a high full-to-empty capsid ratio, optimisation strategies include:
- Enhancing transfection efficiency to improve vector genome packaging.
- Optimising harvest timing to minimise incomplete capsid formation.
- Employing gradient ultracentrifugation or chromatography for effective separation.
Other Impurities and Considerations
Beyond capsid impurities, additional contaminants such as host cell proteins, residual DNA, and process-related impurities must be minimised. ELISA and qPCR are commonly used to quantify these contaminants to meet regulatory safety requirements.
Capsid Aggregation: Another critical impurity, capsid aggregation, can significantly impact AAV stability, safety, and transduction efficiency. Aggregation assessment and control strategies will be further discussed in the later section – Stability and Storage.
2. Genomic Integrity and Titer
Ensuring accurate AAV genome integrity and titer is critical for the potency and consistency of gene therapy products. The viral genome must be intact and properly packaged within the capsid to enable efficient transduction and therapeutic gene expression. Additionally, determining viral titer at different production stages is essential for quality control, process optimisation, and dose determination.
Genomic Integrity
AAV vectors are designed to deliver therapeutic transgenes, and maintaining the integrity of the vector genome is crucial for efficacy. However, genome truncations, rearrangements, or the presence of partial genomes can compromise functionality. Factors affecting genomic integrity include:
- Vector design: Improperly designed constructs can lead to recombination events or truncated genomes.
- Packaging efficiency: Overloading the AAV capsid with oversized genomes (>4.7 kb) can lead to fragmented or improperly packaged genomes.
- Shear forces during processing: Certain purification steps, such as ultracentrifugation and chromatography, may cause genome degradation.
Methods for Assessing Genomic Integrity
- Southern blotting: Detects genome truncations and rearrangements by hybridizing labelled probes to specific sequences within the AAV genome.
- Next-generation sequencing (NGS): Provides high-resolution analysis of genome integrity, identifying mutations, deletions, or rearrangements.
- Pulse-field gel electrophoresis (PFGE): Separates full-length and fragmented genomes based on size.
- Droplet digital PCR (ddPCR): Offers absolute quantification of intact vector genomes, helping to detect partially packaged or fragmented genomes.
Virus Titer
Viral titer quantification is essential for assessing product consistency and determining the appropriate dosage for clinical applications. There are three primary types of AAV titers:
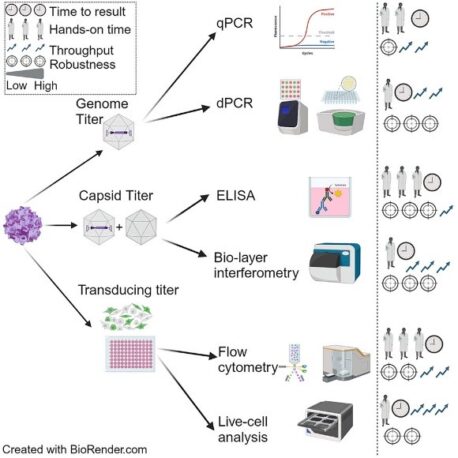
- Genome Titer
Genome titer quantifies AAV particles containing a complete vector genome, serving as a direct measure of vector potency. It is reported in genome copies per milliliter (GC/mL) or vector genomes per milliliter (vg/mL). Key methods include:
- Quantitative PCR (qPCR): A widely used, sensitive method for detecting and quantifying AAV genomes. However, it may introduce variability due to amplification efficiency differences.
- Droplet digital PCR (ddPCR): Provides absolute quantification with higher precision than qPCR, reducing variability and improving reproducibility.
- Capsid Titer
Capsid titer measures the total number of viral particles, regardless of whether they contain a genome. It is crucial for assessing batch consistency and purification efficiency. Common methods include:
- Enzyme-linked immunosorbent assay (ELISA): Uses antibodies targeting capsid proteins to quantify AAV particles with high specificity.
- Biolayer Interferometry (BLI): A label-free, real-time detection method that measures AAV capsid concentration by monitoring binding interactions between viral particles and immobilized antibodies.
Capsid titer is expressed in viral particles per milliliter (vp/mL). Comparing capsid and genome titers helps determine the full-to-empty capsid ratio, a key indicator of vector quality.
- Transducing / Infectious Titer
Not all AAV particles are capable of successfully transducing cells and delivering the therapeutic gene. Infectious titer measures the proportion of functional viral particles, typically reported as infectious units per milliliter (IU/mL). Common methods include:
- Tissue Culture Infectious Dose (TCID50) assay: Determines the viral dose required to achieve infection in 50% of cultured cells.
- Flow cytometry-based detection: Cells are transduced with AAV encoding fluorescent proteins or tagged viral proteins, and fluorescence intensity is quantified using flow cytometry. This approach is particularly useful for rAAVs designed to express fluorescent markers.
- Live-cell analysis: Live-cell imaging can assess transducing titer by capturing real-time microscopic images of cells before and after transduction. This method provides dynamic insights into transgene expression and cell physiology.
By systematically evaluating genomic integrity and titer across production and quality control stages, manufacturers can ensure that AAV preparations maintain potency, safety, and batch-to-batch consistency.
3. Potency and Transduction Efficiency
Potency refers to the ability of an AAV vector to deliver and express the transgene in target cells. High potency ensures efficient gene transfer and therapeutic effect, making it a key quality control attribute.
Assessing Potency and Transduction Efficiency
To evaluate AAV potency, in vitro transduction assays are commonly used, where AAV vectors infect target cells, and transgene expression is measured. The most widely used methods include:
1. In Vitro Transduction Assays (Cell-Based Assays)
These assays involve infecting cultured cells with AAV and measuring transgene expression. Key factors include:
- Vector dose-response – Higher potency results in stronger gene expression at lower doses.
- Cell type specificity – AAV serotypes exhibit different tropisms, meaning some serotypes transduce specific cell types more efficiently than others.
2. Reporter Gene Expression Analysis
Reporter genes are often used to assess transduction efficiency. Analytical methods include:
- Flow Cytometry:
- Measures fluorescence intensity from reporter genes (e.g., GFP, RFP) in individual cells.
- Allows high-throughput quantification of transduced vs. non-transduced cells.
- Fluorescence Microscopy:
- Provides qualitative and semi-quantitative visualisation of transgene expression.
- Useful for assessing intracellular localisation and vector distribution.
- qPCR or ddPCR:
- Detects vector genome copies inside transduced cells.
- ddPCR offers absolute quantification with higher precision.
- Western Blot or ELISA:
- Used for detecting and quantifying the transgene protein product.
Potency assays are critical for batch-to-batch consistency, ensuring that AAV vectors maintain effective gene delivery.
4. Stability and Storage Considerations
Aggregation Risk and Long-Term Stability
AAV stability is essential for maintaining efficacy, safety, and shelf life. Aggregation of AAV particles can lead to:
- Reduced transduction efficiency – Aggregates impair the ability of AAV particles to enter target cells.
- Altered biodistribution – Aggregates may lead to unwanted accumulation in certain tissues.
- Increased immunogenicity – The immune system may recognise aggregated AAV as a foreign threat.
Analytical Methods for Monitoring Aggregation
- Dynamic Light Scattering (DLS):
- Measures the size distribution of AAV particles in solution.
- Detects early-stage aggregation before visible precipitation occurs.
- Size-Exclusion Chromatography (SEC):
- Separates and quantifies aggregated vs. monomeric AAV particles.
- Often coupled with multi-angle light scattering (SEC-MALS) for accurate size determination.
Storage Buffer Optimisation
AAV stability depends on formulation buffers that prevent degradation. Key considerations include:
- pH and Ionic Strength: AAV is stable within a narrow pH range (~pH 7.5-8.0); deviations may cause capsid instability.
- Surfactants: Adding surfactants (e.g., Pluronic F-68) helps prevent aggregation.
- Cryoprotectants: Sugar-based cryoprotectants (e.g., trehalose, sucrose) improve stability during freezing.
- Avoiding Freeze-Thaw Cycles: Repeated freezing and thawing can lead to capsid damage and loss of potency.
Proper formulation and storage conditions ensure that AAV vectors retain functionality over time, reducing manufacturing waste and ensuring consistent therapeutic performance.
5. Sterility and Safety Testing
Ensuring sterility and absence of contaminants is critical for clinical-grade AAV products. Contaminants such as mycoplasma, endotoxins, and adventitious agents pose significant safety risks and must be rigorously tested.
Mycoplasma and Endotoxin Testing
- Mycoplasma Testing:
- Mycoplasma contamination can alter AAV potency and cell viability.
- PCR-based assays and culture-based methods (e.g., broth or agar cultures) are commonly used for detection.
- Endotoxin Testing:
- Endotoxins (lipopolysaccharides from Gram-negative bacteria) can trigger severe immune reactions.
- Limulus Amebocyte Lysate (LAL) assay is the gold standard for endotoxin quantification.
Adventitious Agent Screening
Adventitious agents are unintended viral or microbial contaminants that may arise during cell culture and vector production. Testing strategies include:
- PCR-based methods: Detect known viral contaminants in AAV production systems.
- Electron Microscopy: Used to identify unknown viral contaminants.
- NGS: Provides high-throughput screening for unexpected microbial and viral contaminants.
By implementing robust sterility and safety testing, manufacturers ensure that AAV vectors meet regulatory standards and minimise risks for patients receiving gene therapies.
Quality control in AAV manufacturing is multifaceted, covering everything from genome integrity and potency to safety and stability. By implementing stringent analytical techniques and optimised processes, researchers and manufacturers can ensure the production of high-quality, safe, and effective AAV vectors for gene therapy applications.
Leveraging PackGene for AAV Design, Production, and Quality Control
For researchers and companies looking to streamline AAV vector development, outsourcing to PackGene offers a comprehensive solution from gene synthesis to final quality control. PackGene provides expert assistance in AAV plasmid cloning, including access to their GOI library for optimised gene expression and tissue-specific promoter selection.
Beyond vector design and production, PackGene has established multiple advanced AAV analytical platforms to ensure rigorous quality control. In addition to standard assessments such as toxicity, genomic titer, and purity, they offer specialised assays for titration, genome integrity, characterisation, aggregation, contamination, and safety. These evaluations provide a thorough assessment of AAV vectors, ensuring compliance with the highest standards for safe and effective gene therapy applications.
Partner with PackGene to accelerate your AAV research and production. Contact Atlantis Bioscience today to discuss your project needs and explore customised solutions tailored to your gene therapy goals.
References
Kontogiannis T, Braybrook J, McElroy C, Foy C, Whale AS, Quaglia M, Smales CM. Characterization of AAV vectors: A review of analytical techniques and critical quality attributes. Mol Ther Methods Clin Dev. 2024 Jul 30;32(3):101309. doi: 10.1016/j.omtm.2024.101309.
Meierrieks F, Kour A, Pätz M, Pflanz K, Wolff MW, Pickl A. Unveiling the secrets of adeno-associated virus: novel high-throughput approaches for the quantification of multiple serotypes. Mol Ther Methods Clin Dev. 2023 Sep 20;31:101118. doi: 10.1016/j.omtm.2023.101118.
Pillay S, Carette JE. Host determinants of adeno-associated viral vector entry. Curr Opin Virol. 2017 Jun;24:124-131. doi: 10.1016/j.coviro.2017.06.003. Epub 2017 Jun 30.
Pipe S, Leebeek FWG, Ferreira V, Sawyer EK, Pasi J. Clinical Considerations for Capsid Choice in the Development of Liver-Targeted AAV-Based Gene Transfer. Mol Ther Methods Clin Dev. 2019 Sep 10;15:170-178. doi: 10.1016/j.omtm.2019.08.015.
Wagner C, Fuchsberger FF, Innthaler B, Lemmerer M, Birner-Gruenberger R. Quantification of Empty, Partially Filled and Full Adeno-Associated Virus Vectors Using Mass Photometry. Int J Mol Sci. 2023 Jul 3;24(13):11033. doi: 10.3390/ijms241311033.
Wang, JH., Gessler, D.J., Zhan, W. et al. Adeno-associated virus as a delivery vector for gene therapy of human diseases. Sig Transduct Target Ther 9, 78 (2024). https://doi.org/10.1038/s41392-024-01780-w
Suoranta T, Laham-Karam N, Ylä-Herttuala S. Strategies to improve safety profile of AAV vectors. Front Mol Med. 2022 Nov 1;2:1054069. doi: 10.3389/fmmed.2022.1054069.
Xu, L., Yao, S., Ding, Y.E. et al. Designing and optimizing AAV-mediated gene therapy for neurodegenerative diseases: from bench to bedside. J Transl Med 22, 866 (2024). https://doi.org/10.1186/s12967-024-05661-2
CONTACT

QUESTIONS IN YOUR MIND?
Connect With Our Technical Specialist.

KNOW WHAT YOU WANT?
Request For A Quotaiton
OTHER BLOGS YOU MIGHT LIKE
HOW CAN WE HELP YOU? Our specialists are to help you find the best product for your application. We will be happy to help you find the right product for the job.

TALK TO A SPECIALIST
Contact our Customer Care, Sales & Scientific Assistance

EMAIL US
Consult and asked questions about our products & services

DOCUMENTATION
Documentation of Technical & Safety Data Sheet, Guides and more..
