Description:
Human serum albumin (HAS) is the most abundant protein in human plasma, and an attractive macromolecular carrier due to its availability in pure form and its biodegradability, nontoxicity and non-immunogenicity. It has a serum half-life of approximately 20 days, and is widely used as a stabilizing component in pharmaceutical and biologic products, such as vaccines, cell and gene therapies and coatings for medical devices. Due to the potential existence of various infectious disease pathogens in the human blood, especially AIDS, hepatitis B and other viruses, these virus may be brought with HSA to the final products.
HSA is also an important supplement in cell culture medium. It can transport important nutrients to cells, increase the viscosity of the medium, and provide a mechanical protection for cells. Moreover, HSA binds toxins thus avoiding toxic effects, binds excessive proteins acting as a buffer.
Benefits of using Atlantis Bioscience’s recombinant human serum albumin:
- Promotes cell growth and differentiation
- Supports cell survival in a variety of culture systems
- Reduces the need for animal-derived serum
- Is a cost-effective alternative to bovine serum albumin
In recent years, there has been an increased demand for animal-free components to replace serum or animal originated supplements to eliminate the risk of viruses and mycoplasma contaminants. Using the rice endosperm specific expression platform, the recombinant human serum albumin (OsrHSA), which is animal component free, can provide an economical and safe approach for the production of non–animal-derived compounds, which will facilitate development of products from cell cultures.
Also, compared to plasma human serum albumin (pHSA), OsrHSA has the same second and tertiary structure, physiological and biochemical properties with pHSA, while higher purity and excellent batch consistency.
| Source | Rice Grain (Oryza Sativa) | Rice Grain (Oryza Sativa) |
| Product | Cell Culture Grade | Excipient Grade |
| Catalog Number | HYC002M01 | HYC002C02 (Liquid) |
| Formulation | Lyophilized powder | Liquid |
| Package Size | 1g, 10g, 50g and 100g | 50ml with 20% concentration |
| Endotoxin | Less than 0.125EU/mg | Less than 0.167EU/ml |
| Physical Appearance | Off-white to light beige lyophilized powder |
Slightly sticky, yellow or brown transparent liquid |
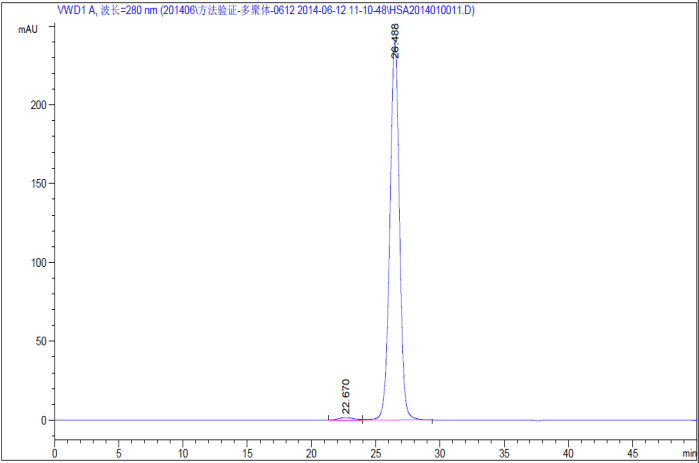
| Purity | More than 99% as determined by SDS-PAGE analysis | More than 99% as determined by SDS-PAGE and HPLC analysis |
| DMF No. | 029648 |
Quality Control Tests:
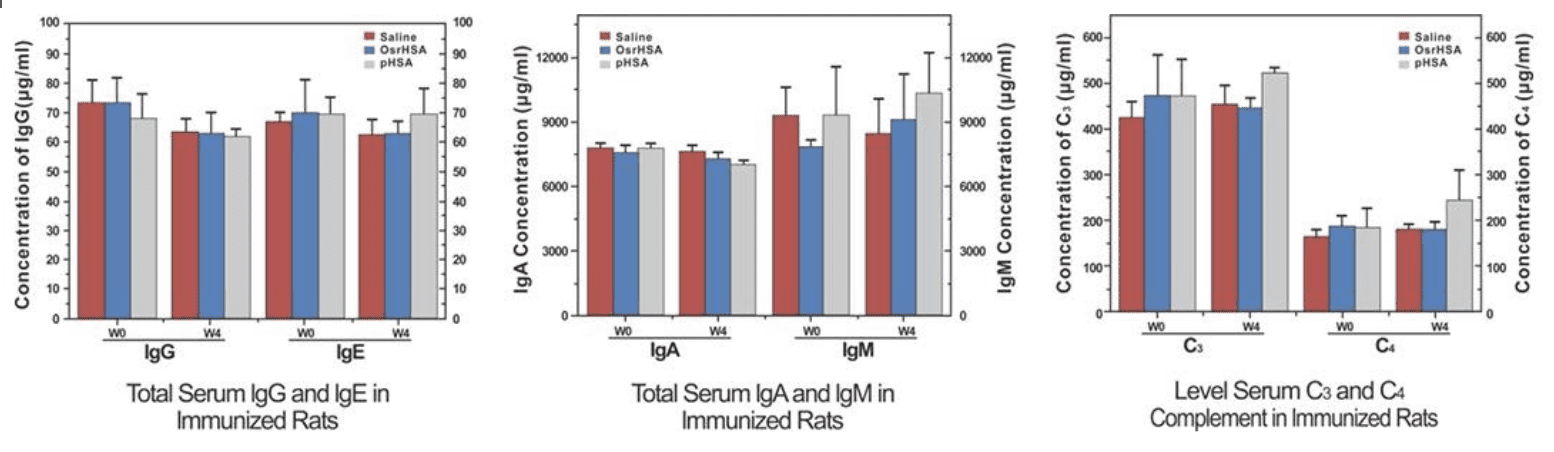
- OsrHSA has equal safety in vivo to pHSA
The titers of individual immunoglobulins IgG, IgM, IgE, and IgA showed no significant difference among the pHSA, OsrHSA, and saline groups, and the titers of C4 complement were not significantly different.
- OsrHSA maintains iPSC viability and stemness
The immunofluorescent staining showed OsrHSA was effective in keeping undifferentiation and keeping viability of iPS cells.

- Performance of OsrHSA in CIK cell therapy
OsrHSA was used in PBMC culture to induce the CIK cells. After 14 days, the total cell amount reach to 6.6X109, and the CD3+CD56+ rate is more than 22.97%. It showed that OsrHSA had effective action in promoting CIK cell growth.

Applications
- Cell & gene therapy development and optimizing cell therapy performance
Cell therapies, including immune cell and stem cell-based therapies, are some of the most sophisticated therapeutics in development. Cell therapy offers an alternative approach to more widely used treatment options. However, the area faces challenges on several fronts, such as upscaling, logistics and ensuring consistent performance. Consequently, there is a need to optimize cell performance to achieve a viable cell product and safe therapeutic solution to patients. - Recombinant human albumin for stem cell culture, cryopreservation & formulation
Albumin is well known to facilitate growth of many cell types, such as mesenchymal stem cells (MSC’s), embryonic stem cells (ESC’s), induced pluripotent stem cells (iPSC’s) and immune cells. Today, several of albumin’s biological properties have been proven valuable across the cell therapy value chain, including:
-
-
- Transportation and complexation with metals or other molecular entities, creating an optimal micro-environment for sustained cell viability.
- Acting as a rich nutrient source ensuring optimal conditions, particularly during cell proliferation.
- Functioning as a pH buffer to prevent unwanted effects during differentiation.
- Maintaining cell viability during cryopreservation – driven by the high purity of our recombinant albumin.
- Acting as a scavenger of toxins and other reactive oxygen species, albumin protects cells against chemical stress all the way from culture to patient.
- Providing an insulation effect in media due to its propensity to distribute evenly throughout solutions.
- Vaccine stabilization
-
- Maintaining vaccine Vaccines stability continue to be an important field of pharmaceutical research, offering valuable therapeutic and prophylactic patient benefits. Like other biologics, vaccines are susceptible to environmental stress, such as temperature changes and physical strain. Therefore, maintaining stability during manufacturing, storage and delivery of vaccines to patients can be a difficult task. The ability to maintain stability in such conditions lead to reduction in cost and more effective vaccination programs to people worldwide. Even when freeze-drying is chosen as a formulation option, reduction of cell viability and vaccine efficacy can occur for temperature sensitive vaccines.
Overcoming vaccine challenges using albumin
-
- Protection from sheer stress: Recombinant human albumin provides an insulating effect on viral vector vaccines, protecting against physical damage during manufacturing and handling, ultimately maintaining potency and efficacy.
- Prevention of surface adsorption: Loss of vaccine particles on surfaces during downstream processing can cause significant loss of yield. Furthermore, adsorption to final vaccine containers may result in inadequate dose control. By coating surfaces with our recombinant HSA, these issues may be prevented.
- Thermal stability: Thermally sensitive vaccines can lose viability and efficacy during freeze-thaw and/or fluctuating temperatures under transportation. Driven by albumin’s high thermal stability, our recombinant HSA has shown to extend this quality to co-formulated vaccines.
- These properties have been shown to be applicable across a broad range of vaccine constructs, such as live attenuated vaccines, whole-inactivated, sub-unit vaccines, viral vector vaccines, virus like particles (VLP’s) and enveloped vaccines.
- Protein & peptide formulation Given the instable nature of proteins and peptides, optimizing the stability of such therapeutics is a critical yet challenging objective of any drug development program. Resolution of such challenges enables their full therapeutic potential to be exploited, thereby ensuring optimal treatment options.
Formulation challenges
Safety and activity can be impaired by loss of “integrity” of the formulated product. Proteins and peptides indeed are only marginally stable and can undergo degradation via several mechanisms:
-
- Hydrophobic interactions and peptide fibrillation may lead to aggregates and sub-visible particles, resulting in reduced efficacy and increased immunogenicity.
- Binding to surfaces can cause structural changes resulting in self-association and/or aggregation. Further to this, non-specific adsorption to drug containers may cause material loss and incorrect dosing.
- Chemical stress, such as degradation by oxidative species, can result in a range of functional changes such as altered binding activities, increased susceptibility to aggregation and proteolysis, and increased or decreased uptake by cells, as well as altered immunogenicity.
Choosing the right formulation strategy
Companies often work within a standard toolbox of excipients for formulation development. Although many of these agents are effective stabilizers, their use requires careful consideration in terms of local toxicity and potential immunogenicity. Often the outcome is a cocktail of excipients. In some instances, it is quite difficult to combine the various “ingredients” to reach the levels of stabilization needed. As formulation becomes increasingly complicated to achieve, there is an equally increased need for advanced solutions.
Albumin is nature’s own stabilizer, with in vivo functions to transport and protect vital molecules. Our recombinant human albumin exploits albumin’s multifunctional properties as an effective, versatile stabilizer. It is particularly suited as a leaner formulation approach of biological drugs, such as proteins or peptides, not readily stabilized using traditional formulation strategies.
- Medical device coating
Biocompatible medical device coating using recombinant human albumin
Innovation in medical devices continues to create novel treatment options. However, medical device developers are facing challenges in ensuring safe and biocompatible products. Biocompatibility is crucial when using medical devices to ensure they do not elicit an adverse response by the patient.
Due to its natural propensities, albumin is widely utilized in the medical device industry for coating of stents and tubing, for instance in dialysis, to improve their biocompatibility.
Albumin’s natural surface coating ability
Albumin has been demonstrated to cover both hydrophobic and hydrophilic surfaces in nearly a single molecule layer. This means that even small amounts of albumin can effectively cover large areas of surface. By coating with albumin, a biological interface is created, thereby limiting the exposure of the otherwise incompatible surface to the patient, increasing product safety.
The improved purity and defined nature of our product consistently produces the same surface coverage. This makes our product especially suited to the medical devices industry as it minimizes the need for elaborate release procedures and product performance monitoring. Additionally, due to its recombinant nature, is free of risk with respect to human derived pathogens and viruses, further warranting a safe product.
Reference Publications
Wang, L., Wang, X., Wu, Y., Wang, J., Zhou, W., Wang, J., Guo, H., Zhang, N., Zhang, L., Hu, X., Zhao, Y., Miao, J., Zhang, Z., Dunmall, L. S. C., Zhang, D., Lemoine, N. R., Cheng, Z., & Wang, Y.
A novel microenvironment regulated system CAR-T (MRS.CAR-T) for immunotherapeutic treatment of esophageal squamous carcinoma.
Cancer Letters, 568, 216303. (2023).
DOI: https://doi.org/10.1016/j.canlet.2023.216303
Article Snippet: “To demonstrate effective binding between HSABP and HSA, we labelled 1 mg plant origin HSA (Oryzogen, HYC002M01) with FITC to facilitate detection. HSA-starved T cells, B7-H3.CAR-T cells and MRS.B7-H3.CAR-T cells were incubated with HSA-FITC at 4 °C for 30min, 20min and 10min respectively.”
Hao, C., Chu, S., Quan, X., Zhou, T., Shi, J., Huang, X., Wu, G., Tortorella, M. D., & Pei, D.
Establishing extended pluripotent stem cells from human urine cells.
Cell & Bioscience, 13(1). (2023).
DOI: https://doi.org/10.1186/s13578-023-01051-1
Article Snippet: “TSCM2 medium consists of: 1 μM PD0325901 … (Oryzogen, HYC002M01), 32.7 μM Ethanolamine (Selleck, S6210), GlutaMAX-I, Sodium pyruvate.”
Zhao, P., Sun, T., Lyu, C., Liang, K., Niu, Y., Zhang, Y., Cao, C., Xiang, C., & Du, Y.
Scar‐Degrading endothelial cells as a treatment for advanced liver fibrosis.
Advanced Science, 10(4). (2022).
DOI: https://doi.org/10.1002/advs.202203315
Article Snippet: “Liver-targeting peptides (fpps, GQLKHLEQQEG)[23] were dissolved in DMEM medium containing 1% PS, 1× GlutaMax (Thermo Fisher, 35 050 061), and 0.5 mg mL−1 albumin (oryzogen, HYC002M01).”
李天晴 & 冯春
一种间充质干细胞的培养方法
Yunnan Key Lab Of Primate Biomedicine Research, (2020).
https://patents.google.com/patent/CN112522191A/zh
Article Snippet: “…可通过市售途径获得符合上述要求的重组人白蛋白,比如禾元生物,HYC002M01。”