- Your cart is empty
- Continue Shopping
Custom mRNA and Lipid Nanoparticles Services (GMP Available)
Looking for reliable and high-quality custom mRNA services for your gene therapy research?
We have partnered with PackGene, a specialist in RUO (Research Use Only) and GMP (Good Manufacturing Practice) grade mRNA production, tailored to meet the unique needs of researchers across Southeast Asia.
Why Choose Our Custom mRNA Services?
mRNA (messenger RNA) is revolutionizing therapeutic development as a cornerstone for vaccines, cancer treatments, and genetic disorder therapies. With its ability to enable localized expression, rapid development cycles, and unparalleled safety as a non-genome-editing platform, mRNA is driving innovation in modern medicine.
At PackGene, we provide:
- Custom mRNA manufacturing for applications like EGFP mRNA, mCherry mRNA, and other research-grade molecules.
- Seamless transition from RUO-grade mRNA production to GMP-grade mRNA manufacturing, ensuring clinical readiness.
- Industry-leading timelines and exceptional quality backed by rigorous quality control protocols.
Leverage PackGene’s expertise to streamline your mRNA production process, from early research to scalable GMP manufacturing. Let us help you accelerate breakthroughs in gene therapy and achieve impactful results.

Superior Quality
mRNA quality is ensured with CE testing, along with guaranteed LNP uniformity and a comprehensive QC panel.

One-stop mRNA solution
Offering on-shelf and custom mRNA solutions, mRNA-to-LNP services, and seamless transition from RUO to cGMP production.
Our mRNA
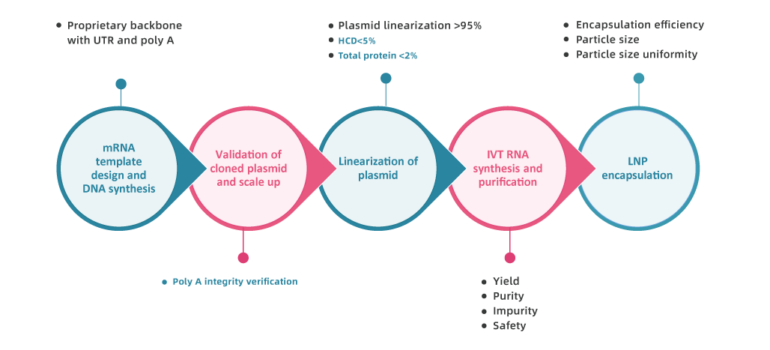
How is our mRNA made?
Custom mRNA production using in vitro transcription (IVT) is a proven method for synthesizing high-quality RUO mRNA and GMP mRNA from plasmid DNA templates. PackGene’s IVT process incorporates a promoter sequence, the gene of interest, and a poly(A) tail, streamlining mRNA manufacturing while ensuring consistency, scalability, and cost-effectiveness for research, preclinical, and clinical applications.
Key Advantages of IVT for mRNA Production:
- Simplified Workflow: The poly(A) tail is directly incorporated during IVT, eliminating additional post-transcription steps, which reduces production time.
- Higher Efficiency: Incorporating the poly(A) tail during IVT ensures more efficient polyadenylation, enhancing mRNA stability and translation efficiency.
- Consistency: Plasmid templates with pre-designed poly(A) tails ensure uniform length and composition across all mRNA products, improving batch-to-batch reproducibility.
- Scalability: Once a plasmid template is established, mRNA production can be easily scaled to meet research or clinical needs.
- Customization: Plasmids can be engineered with specific regulatory elements like promoters and enhancers to optimize mRNA expression and stability for various applications.
- Cost-Effectiveness: Plasmid-based IVT eliminates the need for costly poly(A) tailing enzymes, making it an economical option for high-quality mRNA manufacturing.
What We Offer
| Catalog | Capping and modification | Gene | Quantity | Timeline (Business days) |
|---|---|---|---|---|
| mRNA | Custom mRNA Cap1 | Custom gene | 100μg-20mg | 10-15 |
| mRNA | Custom mRNA Cap1 N1meΨU | Custom gene | 100μg-20mg | 10-15 |
| mRNA-LNP | Custom mRNA Cap1 in LNP | Custom gene or off-the-shelf | 100μg-20mg | 10-25 |
| mRNA-LNP | Custom mRNA Cap1 N1meΨU in LNP | Custom gene or off-the-shelf | 100μg-20mg | 10-25 |
*Additional time (~2-3 weeks ) for custom gene synthesis
mRNA
mRNA Grade and QC Standard
| QC Category | QC Item | Method | Specification | Research Grade | Add-on |
|---|---|---|---|---|---|
| Identification | Appearance | Visual Inspection | Clear and free of foreign particles | √ | |
| mRNA Concentration | UV Absorbance by Nanodrop | ≥1mg/ml | √ | ||
| RNA Integrity/size | Capillary Electrophoresis | Target ±30%, Expected band size detected | √ | ||
| Buffer Specification | Client Spec | RnaseFree H2O(default), PBS, 1mM Sodium Citrate, pH 6.4 | √ | ||
| Purity | A 260/280 Ratio | UV Absorbance by Nanodrop | 1.70-2.30 | √ | |
| Size based purity | Capillary Electrophoresis | >80% | √ | ||
| Impurity | Total Protein residue | Nano Orange | ≤1% | √ | |
| Plamsid DNA residue | qPCR | ≤0.1% | √ | ||
| dsRNA | Slot-blot | ≤1% | √ | ||
| Safety | Endotoxin | Semiquantitative LAL | <10EU/mg | √ | |
| Endotoxin | Quantitative | <10EU/mg | √ | ||
| Bioburden | Direct Inoculation | No growth after 48 hrs | √ | ||
| Raw material-Linearization | Linearization percentage | AGE | >95% | √ | HPLC |
| Host cell DNA | Quantitative PCR | ≤ 5% | √ | ||
| Total protein | Nano Orange | ≤1% | √ | ||
| RNA | AGE | Non-detectable by gel electrophoresis at 200ng | √ | ||
| Raw material -circular Plasmid | Gene of Interest | Sanger sequencing | 100% math reference sequence (not including poly A) | √ | |
| Poly A | Sanger sequencing | ≤110A ±5nt, ≤111-125A ±8nt | √ | ||
| Poly A Length | Enzyme digestion and CE | Target ±5% | √ |
In vitro transcribed (IVT) mRNA plays a vital role in the development of mRNA-based therapeutics and vaccines. To guarantee the quality of IVT mRNA, a range of mRNA manufacturing quality tests can be conducted. Below are key tests to evaluate the quality of IVT mRNA products:

Gel Electrophoresis
This technique separates and visualizes RNA fragments by size, confirming mRNA integrity and the absence of degradation or impurities.
Spectrophotometry
This technique measures RNA concentration in a solution to ensure it’s within the desired range and free of contaminants that could affect downstream applications.
Endotoxin Testing
Endotoxins are lipopolysaccharides that can trigger immune reactions in humans. To ensure IVT mRNA safety, endotoxin levels must be measured and kept below a specific threshold.

Sterility Testing
Sterility testing using standard microbiological techniques ensures the IVT mRNA is free of microbial contamination.

Capillary Electrophoresis
This technique measures mRNA purity by separating fragments based on size and charge, determining full-length mRNA percentage and detecting degradation or truncation.
Additional Tests to verify the quality of mRNA
- Total Protein by Nano Orange: This test measures the total protein content in the IVT mRNA sample. The Nano Orange dye binds to proteins, and the fluorescence signal is used to determine protein concentration. It helps ensure the sample is free of protein contaminants that could interfere with downstream applications.
- Plasmid DNA Residue by qPCR: This test detects residual plasmid DNA in the IVT mRNA sample. Plasmid DNA used for mRNA synthesis may remain, potentially triggering an immune response. qPCR is a sensitive method for detecting even trace amounts of plasmid DNA.
- dsRNA by Slot-Blot: This test detects double-stranded RNA (dsRNA) in the IVT mRNA sample. dsRNA, which can form during mRNA synthesis, may trigger an immune response. Slot-blot transfers RNA samples to a nitrocellulose membrane, which is hybridized with a dsRNA-specific probe to quantify dsRNA levels.
These tests ensure the quality, purity, and safety of IVT mRNA, which are crucial for successful mRNA-based therapeutics and vaccines.
Transfection Efficiency of mRNA to cell lines
EGFP mRNA(Cap1)mCherry mRNA(Cap1)
2μg 24hrs after transfection
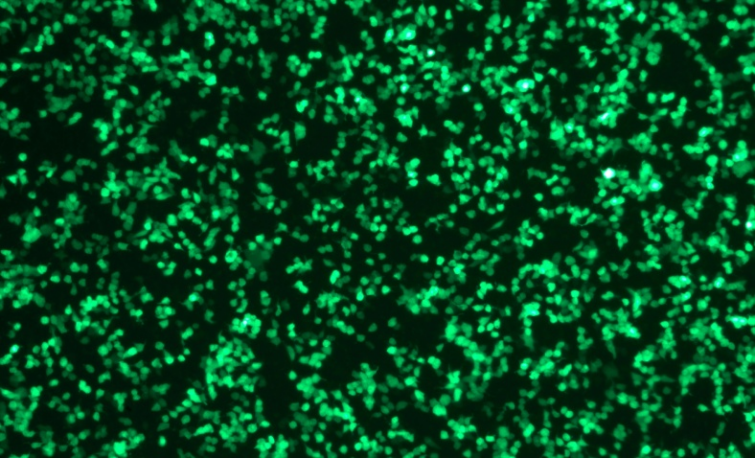
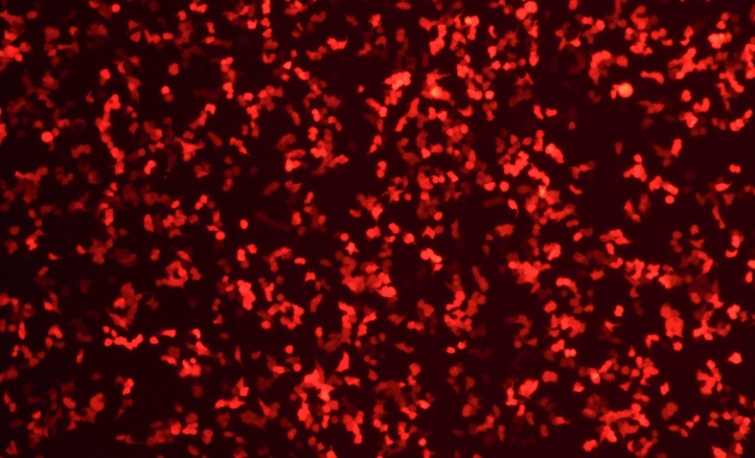
mRNA-Lipid Nanoparticle (LNP)
Lipid nanoparticles (LNPs) are nanoscale lipid-based particles used as delivery vehicles for drugs, including mRNA. LNP-based methods are the most popular for mRNA delivery, offering benefits such as enhanced stability, improved delivery efficiency, reduced immunogenicity, customizability, and a proven clinical success record. These advantages make LNPs a promising tool for the development of safe and effective mRNA-based treatments and vaccines.
Increased Stability
Enhanced Delivery
Reduced Immunogenicity
Customization
Clinical Success
LNPs protect mRNA from degradation by enzymes and other factors in the body, enhancing its stability and improving delivery to target cells.
LNPs enhance the efficient uptake of mRNA by cells, resulting in higher protein expression levels. They also enable targeted delivery of mRNA to specific cells, such as immune or tumor cells, through cell-type specific endocytosis mechanisms.
LNPs can lower the immunogenicity of mRNA by protecting it from the immune system and preventing the activation of innate immune responses. This enhances the safety and efficacy of mRNA-based therapies and vaccines.
The composition of LNPs can be tailored to enhance properties like stability, delivery efficiency, and immunogenicity. This enables customization for specific applications, such as vaccines or therapies aimed at particular diseases.
LNPs have been used successfully in the development of mRNA vaccines for COVID-19, showing high efficacy and safety in clinical trials. This success has increased interest in the use of LNPs for other mRNA-based therapies and vaccines.
mRNA-LNP QC Control
| QC category | QC item | Method | Specification | Research Grade |
|---|---|---|---|---|
| mRNA-LNP (On-shelf and Custom) | RNA Integrity/size | Capillary Electrophoresis | >80% | √ |
| Encapsulate Efficiency | Elisa Analyzer | Report | √ | |
| Particle size | Dynamic Light Scattering | <120nm | √ | |
| Particle Uniformity | Dynamic Light Scattering | <0.2 | √ | |
| Concentration | Capillary Electrophoresis | Encapsulated mRNA <0.2mg/ml | √ |
PackGene ensures the quality, safety, and efficacy of mRNA lipid nanoparticles (LNPs) through key quality control (QC) tests:
Encapsulation Efficiency (EE)
EE measures the percentage of mRNA encapsulated within LNPs, essential for effective delivery.
Encapsulation Efficiency (EE) = (Amount of encapsulated mRNA / Total amount of mRNA) x 100%
For example, if 1 mg of mRNA yields 0.8 mg encapsulated, EE = 80%. High EE ensures optimal therapeutic outcomes.
Particle Size
Particle size impacts biodistribution, cellular uptake, and stability. Larger particles may aggregate, increasing toxicity. Dynamic light scattering (DLS) is commonly used to measure size.
Size Distribution and Uniformity
A narrow size distribution enhances stability and consistency, while variability may lead to toxicity. Methods like DLS and nanoparticle tracking analysis (NTA) assess these parameters.
These tests are critical for ensuring safe, effective LNP-based mRNA delivery.
Case Study
IVT mRNA performance
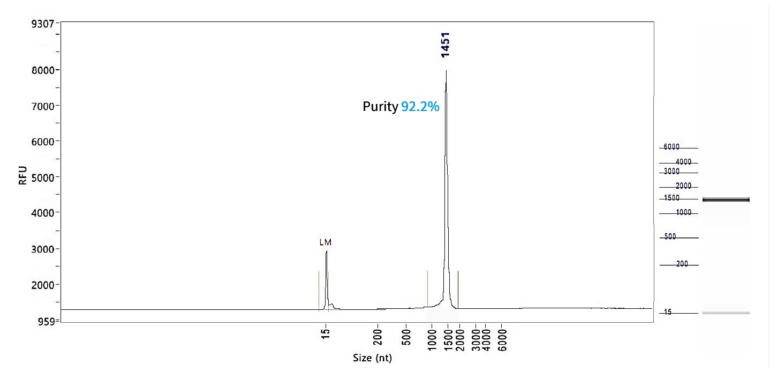
EGFP mRNA (Cap1)
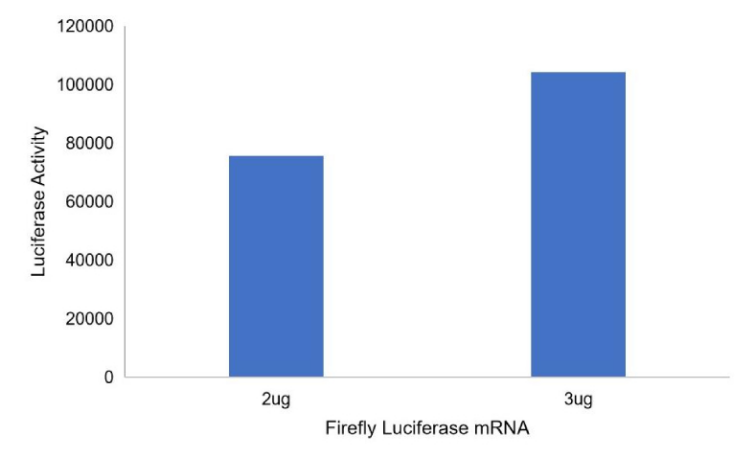
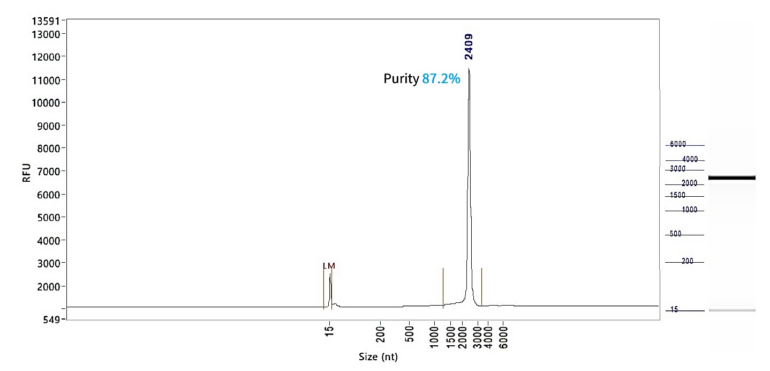
Firefly Luciferase mRNA (Cap1)
mCherry mRNA (Cap1)
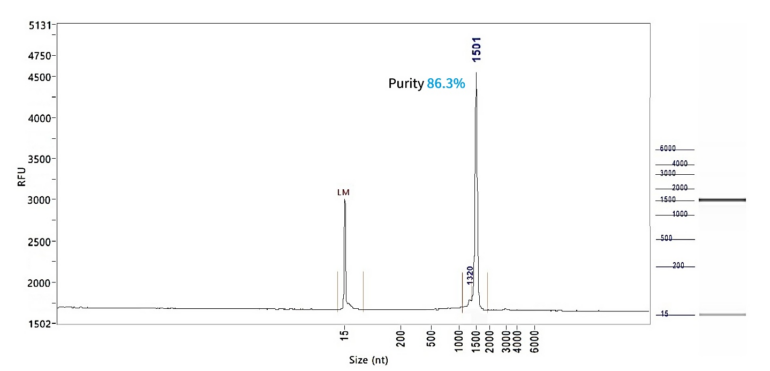
SpCas9 mRNA (Cap1)
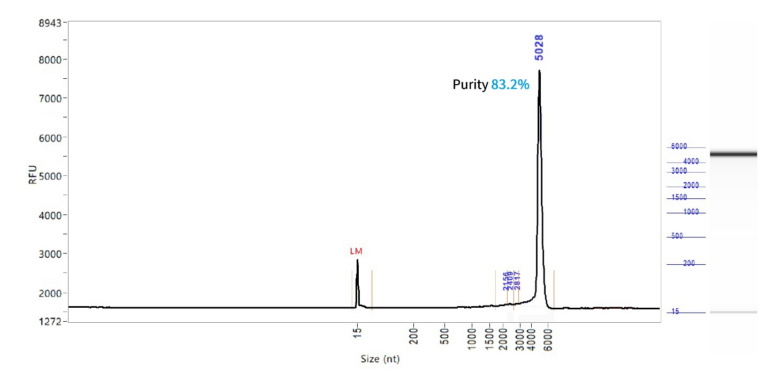
PackGene’s mRNA production demonstrates a size range within ±30% of the target size and a purity level exceeding 80%. Maintaining a ±30% size range ensures that the mRNA is appropriately sized for its intended application and maintains consistency across different sample batches. A purity level above 80% reflects a high concentration of mRNA with minimal contaminants, such as genomic DNA, RNA degradation products, and residual synthesis reagents, which could otherwise compromise the efficacy and safety of the mRNA in downstream applications.
These measurements are performed using the Agilent 5200 CE System, a highly sensitive and precise instrument designed for analyzing nucleic acids, including mRNA.
EGFP mRNA (Cap1) -LNP
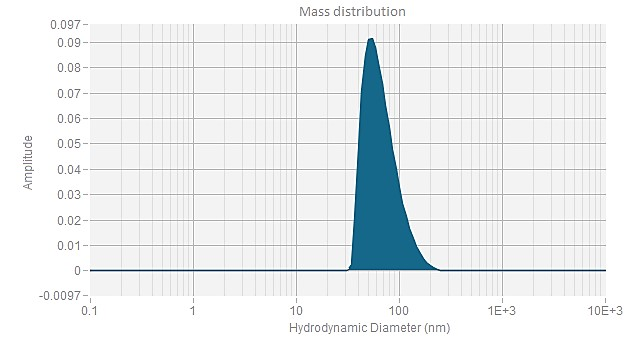
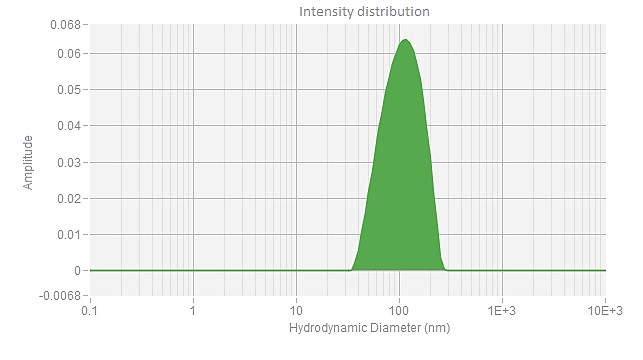
| Z Ave. Dia (nm) | 76.8 |
|---|---|
| PDI | PDI |
| Encapsulation Efficiency | 93.91% |
The Z-average diameter of nanoparticles is determined using dynamic light scattering (DLS), which measures the Brownian motion of particles in solution. By analyzing light scattering fluctuations, the size distribution of the particles is calculated. The Z-average diameter is the intensity-weighted mean size, with larger particles contributing more to the value. This parameter, typically reported in nanometers (nm), reflects the overall size of the particle population. PackGene guarantees an mRNA-LNP Z-average diameter of <120 nm and an encapsulation efficiency of >90%.
The polydispersity index (PDI) measures the size uniformity of nanoparticles. A low PDI value indicates a narrow size distribution, while a high PDI suggests greater size variability. In mRNA delivery, a low PDI is desirable for improved stability, efficacy, and safety. PackGene guarantees a PDI of <0.2 for its mRNA-LNP products, ensuring consistency and high quality.
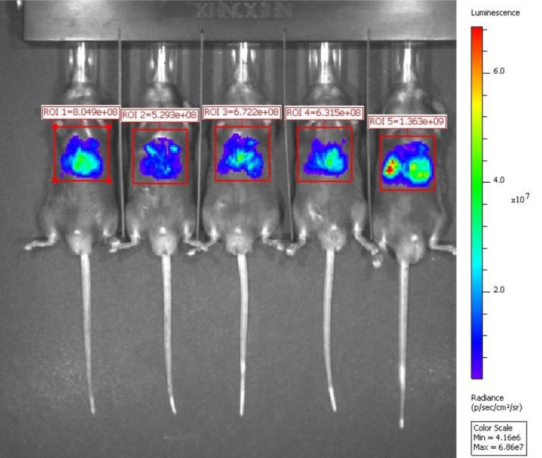
Injection of mRNA-LNP to animals
Firefly luciferase mRNA9(Cap1)-LNP
Live image 6hrs after mouse intravenous injection
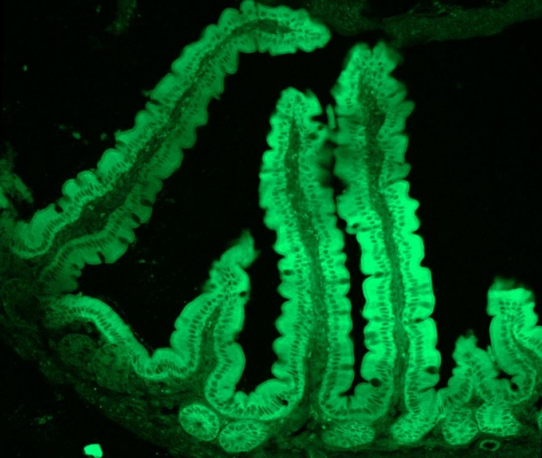
EGFP mRNA9(Cap1)-LNP
Confocal image of mouse intestine frozen section 24hrs after enema
Application

Vaccine Development

Therapeutics

Cellular Programming
mRNA vaccines have gained widespread attention during the COVID-19 pandemic, with vaccines from Pfizer-BioNTech and Moderna receiving emergency use approval. These vaccines work by delivering a small piece of mRNA into the body, which instructs cells to produce a viral protein. The immune system identifies this protein as foreign and triggers an immune response, priming the body to fight the actual virus if encountered in the future.
mRNA also holds potential as a treatment for cancer, genetic disorders, neurodegenerative diseases, and more. In this approach, mRNA is used to introduce new genes into cells, either replacing or supplementing faulty genes responsible for diseases. This technique has shown promise in preclinical studies and is currently being evaluated in clinical trials.
mRNA can also be used to reprogram cells, transforming them into different cell types. This technique has the potential to revolutionize regenerative medicine by enabling the repair or replacement of damaged tissues or organs with healthy cells.
Trusted mRNA Partner in Southeast Asia
PackGene is the trusted partner for researchers working with gene therapy and molecular biology across Southeast Asia, including Singapore, Thailand, Malaysia, and Indonesia. With unparalleled expertise and precision, we provide high-quality mRNA and non viral mRNA-LNP vector production and manufacturing services to accelerate your scientific advancements.
Contact us today to learn how our custom mRNA Production and Manufacturing Services can elevate your research to the next level.
Want To Inquire About The Services?
Contact Us

THE ATLANTIS BIOSCIENCE DIFFERENCE Discover Translational Solutions To Advance From Bench to Bed
GET SUPPORT Whenever You Need It

QUESTIONS IN YOUR MIND?
Connect With Our Technical Specialist.

KNOW WHAT YOU WANT?
Request For A Quotaiton





