- Your cart is empty
- Continue Shopping
Gene Therapy Lentivirus Packaging Service in Southeast Asia
Are you a gene therapy researcher looking to outsource high-quality lentivirus packaging?
PackGene offers a premium lentivirus packaging service optimized for researchers in Southeast Asia, combining proprietary technologies with superior production quality to deliver reliable results.
Key Features
- Third-Generation Lentiviral Vectors: We specialize in 3rd-generation lentiviral packaging systems, which allow for the stable integration of transgenes into host genomes, ensuring long-term expression. These vectors are ideal for gene therapy, stem cell research, and in vivo applications.
- Broad Cell Type Targeting: Our lentivirus packaging services enable the infection of various cell types, including neurons, liver cells, heart muscle cells, tumor cells, and stem cells.
- High Performance: With our optimized packaging process, we enhance the titer, purity, and viability of lentivirus production, delivering consistent and reliable results.
- Self-Inactivating Vector Design: Our third-generation lentiviruses feature a self-inactivating design that reduces the risk of viral replication, ensuring safe and controlled expression.
Highlights

Efficient and Prompt
Delivery time as quick as 8 to 12 business days

Accurate Titer Measurements
Post-transduction qPCR eliminates any overestimation concerns

Professional Technical Support
A response to any inquiries or requests will be delivered within 1 business day

Enhanced Safety
“Self-inactivating” 3rd-generation system increases biosafety
One-stop Solution
From vector design and virus packaging to thorough analysis and testing
Secure Shipping
Dry ice and cold chain logistics to maintain optimal efficacy
Service Details
| Application | Cell culture |
| Quantity | 5E7 TU and up |
| Functional titer (post-transduction qPCR) | ≥1E+8TU/mL |
| Physical titer (p24 or qPCR) | ≥ 2E+9 GC/mL ≥ 1E+10 LP/mL |
| Purification | Crude |
| QC (see add-on QC below) | Post-transduction qPCR, fluorescence images if contain fluorescence |
| Timeline | Start from 7 business days |
| Application | In vivo study |
| Quantity | 5E7 TU and up |
| Functional titer (post-transduction qPCR) | ≥1E+8TU/mL |
| Physical titer (p24 or qPCR) | ≥ 2E+9 GC/mL ≥ 1E+10 LP/mL |
| Purification | Sucrose gradient |
| QC (see add-on QC below) | Post-transduction qPCR, fluorescence images if contain fluorescence |
| Timeline | 3-4 weeks |
- Lentivirus will be offered at a concentration of >1E8 TU/mL
- Yield is not guaranteed for constructs with elements detrimental to the viral packaging process. This includes but is not limited to, toxic genes, genes that disrupt viral packaging, genes that disrupt cell or lentiviral RNA integrity, sequences prone to rearrangement or secondary structures, nucleoproteins, transmembrane proteins, receptor genes, and gene segments with over 6.5kb between LTRs.

Need control virus?
Off-the-shelf (CMV.Gene.EF1.GFP-T2A-Puro.WPRE) are offered at 2E7 TU per vial at ≥1E+8TU/mL. with both in vitro and in vivo grade available.
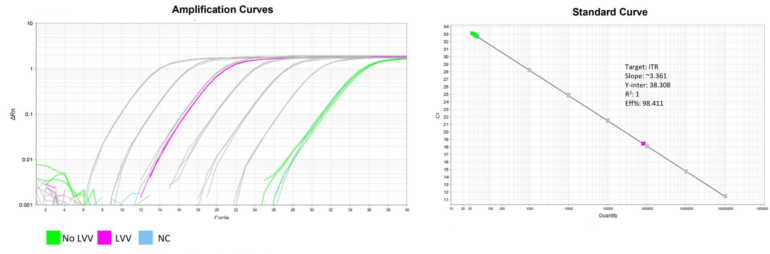
No more concerns of overestimation
Accurate titer measurements by post-transduction qPCR
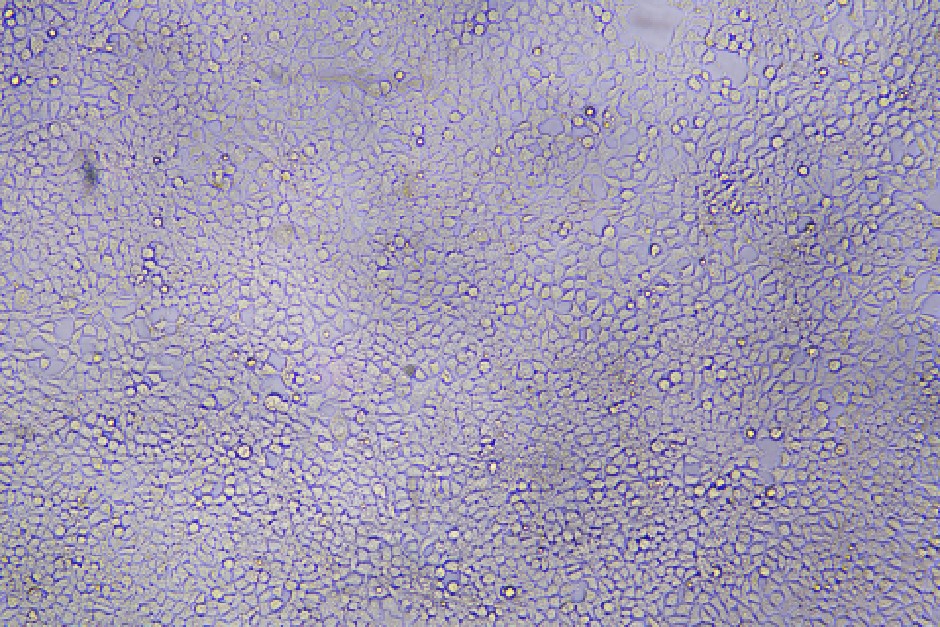
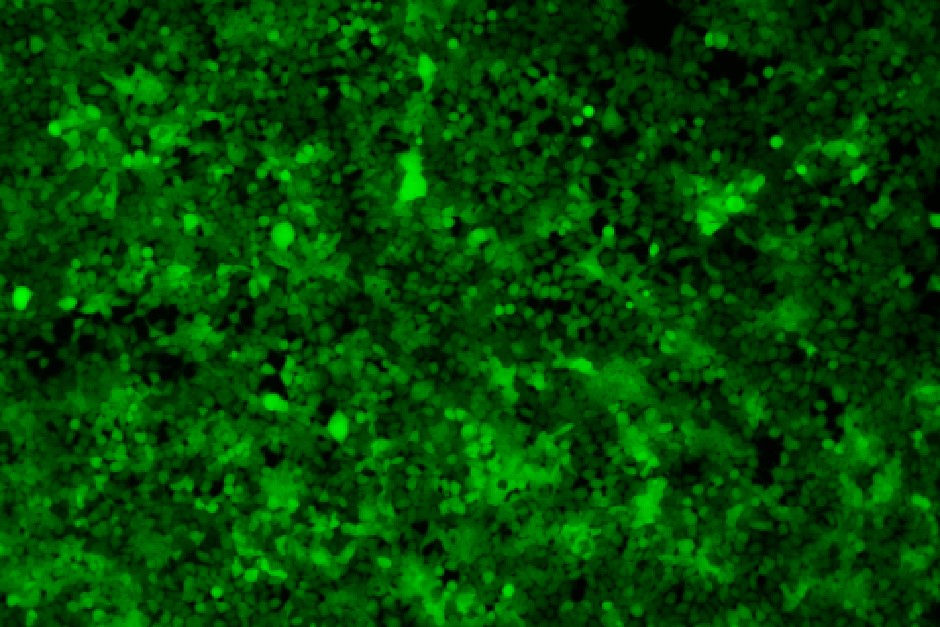
96-well plate, 1.5E+04 cells/well, virus 1.8µL, 72hrs after transduction
Technical Details
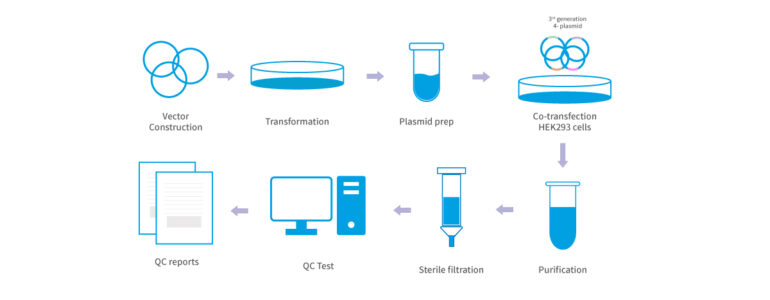
Our end-to-end lentivirus packaging, for gene delivery in research and therapeutic applications begins with vector construction using plasmids that carry the desired genetic material, followed by transformation into bacterial cells for plasmid replication and purification (plasmid prep). Next, the plasmids are co-transfected into HEK293 cells to produce lentiviral particles, which are then purified and sterile-filtered to ensure quality and sterility. The lentivirus undergoes QC testing to validate titer, purity, and transduction efficiency, culminating in final QC reports. Southeast Asia’s expanding biotech sector increasingly provides comprehensive lentivirus packaging services, supporting both research and clinical applications.
Quality Control
| Category | QC test | Description | Release standard |
| Titer | qPCR | qPCR quantifies the number of viral genome copies providing an accurate measure of the viral titer | Report titer |
| Post-transduction qPCR | Infection of cells followed by quantitative qPCR to determine the titer of lentiviruses | Report transduction unit | |
| Transduction test | Gradient dilution of infected cells and count of fluorescent cells | Provide bright field and fluorescent microscopy images | |
| p24 Elisa* | ELISA test for the p24 protein, a core capsid protein of HIV that is used to measure lentivirus titer | Report titer | |
| Contamination | Mycoplasma test* | Measure mycoplasma DNA by PCR | Guaranteed negative |
| Bioburden* | Plate count method: Quantification of live microorganisms (fungi, bacteria, etc.) using colony-forming units (CFUs) | No growth on the agarose plate | |
| Safety | Endotoxin LAL* | Measure endotoxin level by gel clot assay | Report |
*QC with additional charges
Additional QC Information
High-Efficiency Transduction with Post-Transduction qPCR:
Our post-transduction qPCR assay precisely quantifies viral RNA in transduced cells, providing critical insights into gene delivery efficiency. This ensures optimal performance for your research needs.
Transduction Test: This assay involves infecting a sample of cells with lentivirus and assessing transfection efficiency by measuring transgene expression or the expression of a reporter gene, such as GFP (Green Fluorescent Protein). It is a crucial method for evaluating how effectively the lentivirus facilitates transgene expression in target cells.
| Bright-field | Fluorescent field | Bright-field | Fluorescent field | ||
| 96-well plate, 1.5E+04 cells/well, virus 18µL, 72hrs after transduction |  |  | 96-well plate, 1.5E+04 cells/well, virus 1.8E-03µL, 72hrs after transduction |  |  |
| 96-well plate, 1.5E+04 cells/well, virus 1.8µL, 72hrs after transduction |  |  | 96-well plate, 1.5E+04 cells/well, virus 1.8E-04µL, 72hrs after transduction |  |  |
| 96-well plate, 1.5E+04 cells/well, virus 1.8E-01µL, 72hrs after transduction |  |  | 96-well plate, 1.5E+04 cells/well, virus 1.8E-05µL, 72hrs after transduction |  |  |
| 96-well plate, 1.5E+04 cells/well, virus 1.8E-02µL, 72hrs after transduction |  |  | 96-well plate, 1.5E+04 cells/well, virus 1.8E-06µL, 72hrs after transduction |  |  |
p24 ELISA Assay: The p24 antigen, a key component of the HIV-derived lentivirus particle, serves as a marker for viral titer. This test uses ELISA to accurately measure the concentration of p24 particles in your lentivirus preparation, providing a precise evaluation of lentiviral vector quantity.
Mycoplasma Test: Mycoplasma contamination can severely impact cell culture performance and reduce lentivirus production efficiency. This test ensures that cell cultures used for lentivirus packaging are free from mycoplasma, safeguarding the integrity of your viral vectors.
Bioburden Test: Assessing the total viable microorganisms in lentiviral products or on production surfaces, the bioburden test is vital for maintaining sterility and verifying cleanliness throughout the packaging process.
Endotoxin LAL Test: Endotoxins from Gram-negative bacteria can cause inflammatory responses and compromise in vivo applications. The Limulus Amebocyte Lysate (LAL) test is performed to detect and quantify bacterial endotoxins, ensuring the lentivirus is safe for clinical or experimental use.
Lentiviral Transfer Plasmid
PackGene’s lentiviral vectors for gene overexpression are expertly engineered to elevate the expression of specific genes in target cells. These vectors are ideal for investigating gene function, disease modeling, or developing therapeutic strategies where increased gene expression is critical.
- Optimized for High Expression: Each vector features a robust promoter to ensure strong and consistent transgene expression.
- Seamless Integration: Following lentivirus packaging and transduction, the gene of interest integrates into the host cell genome, providing long-term and stable expression.
- Customizable Design: Start with one of PackGene’s ready-to-use LVV transfer plasmid backbones or design your own plasmid, and we’ll synthesize and package it into high-titer lentivirus for your project.
| Vector ID | Promoter | GOI capacity | Promoter for Selection marker | Selection marker |
| XL03C | CMV | 3.3Kbp | EF1 | CopGFP-2A-Puro |
| XL10C | CMV | 4Kbp | EF1 | mRuby2 |
| XL11C | CMV | 4Kbp | EF1 | mCitrine |
| XL12C | CMV | 4Kbp | EF1 | EYFP |
| XL22C | CMV | 3.2kp | EF1 | mCherry-2A-Neo |
| XL23C | EF1 | 4.2kb | T2A | CopGFP |
shRNA Lentiviral Vectors for Precise Gene Silencing
PackGene’s shRNA lentiviral vectors are expertly crafted for effective gene silencing. These vectors express short hairpin RNA (shRNA), which is processed into small interfering RNA (siRNA) within the cell. The siRNA targets and degrades specific mRNA, reducing the expression of the desired gene.
Ideal for gene function studies, shRNA lentiviral vectors enable researchers to knock down gene expression and analyze resulting phenotypic changes. They are also pivotal in therapeutic research, offering potential solutions for diseases where gene downregulation is beneficial.
| Vector ID | Promoter | Promoter for Selection marker | Selection marker |
| XL03B | U6 promoter | EF1a | EGFP-2A-Neo |
| XL04B | U6 promoter | CMV | CopGFP-2A-Puro |
| XL05B | U6 promoter | CMV | Puro |
| XL06B | U6 promoter | PGK | Puro-lRES-mCherry |
| XL07B | H1 promoter | CMV | CopGFP |
| XL08B | H1 promoter | CMV | CopGFP-2A-Puro |
| XL09B | H1 promoter | CMV | Puro |
| XL012B | U6 promoter | CMV | Neo |
Trusted Lentivirus Partner in Southeast Asia
PackGene is the go-to provider for researchers across Southeast Asia, including Singapore, Thailand, Malaysia, and Indonesia. With unmatched expertise and precision, we deliver high-quality lentiviral vectors to accelerate your scientific discoveries.
Contact us today to learn how our lentivirus packaging services can elevate your research to the next level.
Want To Inquire About The Services?
Contact Us

THE ATLANTIS BIOSCIENCE DIFFERENCE Discover Translational Solutions To Advance From Bench to Bed
GET SUPPORT Whenever You Need It

QUESTIONS IN YOUR MIND?
Connect With Our Technical Specialist.

KNOW WHAT YOU WANT?
Request For A Quotaiton





