- Your cart is empty
- Continue Shopping
Gene Therapy AAV Packaging Services in Southeast Asia
Adeno-associated viruses (AAVs) have emerged as a powerful tool for gene therapy due to their unique properties:
Non-pathogenic
AAVs are non-pathogenic, making them safe for therapeutic applications.
Mild Immune Response
AAV infections typically elicit a mild immune response, minimizing side effects.
Broad Tissue Tropism
Different AAV serotypes can target specific tissues and cell types, enabling precise gene delivery.
Long-Term Gene Expression
AAV vectors can achieve long-term gene expression without integrating into the host genome.
Atlantis Bioscience, in partnership with PackGene, delivers top-tier AAV packaging services to advance gene therapy research across Southeast Asia, including Singapore, Thailand, Indonesia, and Malaysia. With expertise and cutting-edge technology, we provide high-titer, pure, and potent AAV vectors tailored to your needs.
Why Choose PackGene for Your AAV Packaging Needs?

- Fast Turnaround Time: Delivery starting from 12-15 business days
- Proprietary Platform: We utilize optimized cell lines and enhanced RC & helper plasmids for maximized virus yield.
- Extensive Serotype Selection: Choose from over 70 AAV serotypes to target various tissues and cell types.
- Unmatched Quality: Our rigorous QC procedures guarantee AAV vectors with high purity, potency, and safety profiles.
- Scalable Solutions: We offer research, NHP, and HT-grade AAVs, as well as GMP-grade manufacturing for clinical applications.
- Customer Satisfaction Guarantee: We are committed to delivering high-quality AAVs on time and within budget. We offer a 5% credit on your next order if we fail to meet your expectations.
0+
Custom AAV Projects Completed
Different Grades of AAV Packaging Services
NHP Grade
Research Grade
HT Grade
- Most stringent QC measures, including multiple purity and potency assays
- Longer turnaround time due to rigorous QC and additional testing
- Highest price
- Pre-clinical studies, clinical trials
- Highest quality, lowest risk, optimal for regulatory submissions
- Comprehensive QC tests, including purity, potency, and endotoxin testing
- Standard turnaround time
- Moderate price
- Most research applications
- Balanced quality and price, suitable for most research applications
- Basic QC tests, including titer and purity
- Fastest turnaround time
- Lowest price
- Basic research, proof-of-concept studies
- Cost-effective solution for rapid prototyping and initial testing
Our AAV Packaging Service Deliverables:
- High-Titer AAV Vectors: Ready for your research experiments.
- Detailed Quality Control Report: Comprehensive documentation of vector quality and performance.
- Plasmid Design and Construction: We can assist with designing and constructing your plasmid containing the gene of interest.
- AAV Vector Production: We utilize state-of-the-art techniques to produce high-quality AAV vectors.
- Comprehensive Quality Control: Our QC assays verify the identity, purity, and potency of your AAV vectors.
- Custom Aliquoting: We can aliquot your AAV vectors to minimize freeze-thaw cycles and ensure optimal viability.
AAV Packaging Service Price and Turnaround
*The indicated titers are guaranteed except when the insert exceeds the packaging capacity (4.7 kb) or if you choose to provide us with your own modified rep/cap plasmid or helper plasmid.
| AAV Packaging Serotypes | Guaranteed Yield (GC)* | Lead Time (Business Days) |
| Normal-yield AAV Serotypes | 2E+12 GC | 12-15 Days |
| 5E+12 GC | ||
| 1E+13 GC | ||
| 2E+13 GC | ||
| 5E+13 GC | ||
| 1E+14 GC | ||
| 2E+14 GC | 18-24 Days | |
| 5E+14 GC | ||
| 1E+15 GC | 30-45 Days | |
| 2E+15 GC | ||
| Low-yield AAV Serotypes (AAV4, 6, etc.) | 2E+12 GC | 12-15 Days |
| 5E+12 GC | ||
| 1E+13 GC | ||
| 2E+13 GC | ||
| 5E+13 GC | ||
| 1E+14 GC | 18-24 Days | |
| 2E+14 GC | ||
| 4E+14 GC |
– For these extremely low-yield AAV serotypes without production data, we are not able to guarantee the final yield or titer specified here.
NHP Grade AAV Packaging
Overcoming the Challenges of NHP Studies
Non-human primate (NHP) studies are essential for evaluating the safety and efficacy of gene therapies. However, these studies can be complex and costly from animal care, housing and maintenance. PackGene offers high-quality NHP-grade AAV vectors to streamline your research and improve your outcomes.
Traditional challenges include:
- High Cost: NHPs are expensive to acquire and maintain.
- Regulatory Hurdles: Strict regulations and ethical considerations can delay study timelines. (Requires significant amount of paperwork and approvals)
- Variable AAV Quality: Low-quality AAV vectors can lead to inconsistent results and compromised efficacy.
- Immune Response: NHPs can mount immune responses to AAV vectors, reducing their therapeutic potential.
Why Choose PackGene’s NHP-Grade AAV Vectors?
Our high-quality NHP-grade AAV vectors are designed to address these challenges and streamline your NHP studies:
- Tailored Production: We offer a comprehensive range of AAV serotypes, including popular choices like 1, 3b, 5, 7, 8, 9, and Rh10. For less common or experimental serotypes, we can conduct small-scale pilot studies to optimize production parameters and ensure consistent yields.
- Rigorous Quality Control: Our stringent quality control measures guarantee the purity, potency, and safety of our NHP-grade AAV vectors. We perform a comprehensive battery of tests, including:
- Enhanced Efficacy & Genome Integrity: Achieve higher levels of gene expression and therapeutic outcomes. Ensuring the correct sequence and structure of the AAV genome.
- Reduced Immune Response: Minimize off-target effects and improve safety by controlling empty capsid rate and endotoxins to minimise the presence of non-functional particles. The absence of microbial contaminants is ensured.
- Improved Reproducibility: Consistent results across different studies.
- Faster Time to Market: Accelerate your research and development timeline.
| AAV type | Scale | Turnaround time |
| Standard capsids (guaranteed yield) | 5E+13 GC~8E+15 GC | Start from 17 business days |
| Custom capsids(by volume) | Up to 100L with 30mL-200mL small scale test | Start from 25 business days |
Our NHP-Grade AAV Production Process:
- Vector Design and Cloning: We design and construct your gene of interest into AAV plasmids.
- AAV Vector Production: We utilize state-of-the-art techniques to produce high-titer NHP-grade AAV vectors.
- Purification and Concentration: We employ a multi-step purification process to remove cellular debris and other impurities.
- Rigorous Quality Control: Our comprehensive QC measures ensure purity, potency, and safety.
- Sterile Filtration and Aliquoting: We sterile filter the AAV vectors and aliquot them into appropriate volumes for storage and use.
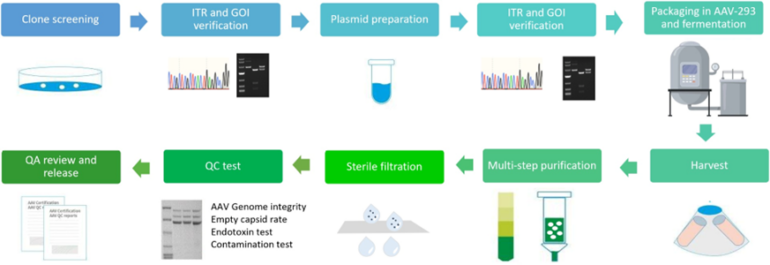
Quality Control Standards of AAV Packaging – NHP Grade specifications
| Test | Method | QC Standard | |
| Standard QC | ddPCR | Measure titer, normalized to 1e13vg/ml | Standard capsids: Concentration and total quantity meet needs. Custom capsids: quantity based on production scale. |
| AAV Capsid size* | SDS-PAGE silver stain | Match capsid protein size | |
| Guarantee endotoxin | LAL | <1EU/ml | |
| Mycoplasma Detection | qPCR | Negative | |
| Bioburden | Direct inoculation | No growth | |
| AAV Genome integrity | CE(titer>1e+12vg/ml, volume >50ul) | Report | |
| Empty Capsid Rate | TEM | <20% | |
| Additional QC | AAV Genome sequencing | Nanopore | Report |
| Empty Capsid Rate | AUC | Report | |
| Endotoxin removal | LAL | <0.2EU/ml | |
| Residual Triton Analysis(bundle with endo removal) | HPLC | 5ppm | |
| Sterility test | Direct inoculation | No growth |
Plasmids used for AAV NHP Grade exceeds industrial standards
To ensure the fidelity of the gene of interest and efficient AAV packaging, the plasmids utilized for NHP grade AAV packaging undergo strict QC. The basic standards consist of requirements for appearance, A260:280 ratio, homogeneity (supercoil >90±10%), restriction analysis, residual RNA and E. coli DNA, endotoxin (≤0.01 EU/µg), and ITR and GOI Sanger sequencing, which are crucial for ensuring the efficient delivery of therapeutic genes via AAV.
In addition to the basic standards, there are also advanced requirements that are not included in the default package and are available upon request with additional cost. These advanced requirements include residual host protein, advanced endotoxin removal, bioburden, mycoplasma contamination, ITR Nanopore sequencing, animal-free production, material archiving, pH (7.5~8.0), residual Kan, USP sterility, dedicated purification resin or filtration membrane, etc.
| Test | Method | QC Standard | |
| Basic standards | Appearance | Visual inspection | Clear, colorless, no visible particulates |
| A260:280 | Nanodrop | 1.8 – 2.0 | |
| Homogeneity | Agarose gel, supercoil >90±10% | Supercoil >90±10% | |
| Restriction Analysis | SmaI/AhdI | Conforming to reference pattern | |
| Residual RNA | Agarose gel | Not visible with 200ng load | |
| Residual E. coli DNA | Agarose gel | <15% of total band with 200ng load | |
| Endotoxin | LAL | ≤0.01 EU/µg | |
| ITR and GOI Sequencing | Sanger | 100% match ITR and GOI correct and clean peak | |
| Additional QC | Residual Host Protein | Nano orange | <0.1% by HCP ELISA |
| Advanced Endotoxin Removal <0.005 EU/µg | Quantitative endotoxin test | <0.005 EU/µg | |
| Bioburden Testing | LB plate | No growth | |
| Mycoplasma Contamination | Quantitative PCR | Negative | |
| Competent cell | JM108, Sure, Sure2, Stbl3, NEBStbl | Customer define | |
| ITR integrity | Nanopore sequencing | Report | |
| Animal Free Production* | TSE/BSE | ||
| Material Archiving | Yes (plasmid+bacteria) | ||
| pH | 7.5~8.0 | ||
| Kan | ELISA | no detectable | |
| USP sterility | Yes | ||
| Dedicated purification resin | Upon request | ||
| Buffer | ddH2O, TE or customer defined | ||
| Dispensing | Upon request | ||
| Dedicated filtration membrane | Upon request |
Performance
The test methods described are necessary to ensure the quality and purity of AAV (adeno-associated virus) produced by PackGene, especially for its use in non-human primates (NHP). The different test methods evaluate various aspects of AAV quality, including its genome integrity, capsid size, and the presence of contaminants.
- ddPCR (droplet digital PCR) is a highly sensitive method for quantifying the amount of AAV in a sample. It allows accurate and precise measurement of AAV genome copies per milliliter.
- qPCR (quantitative PCR) is a method for detecting and quantifying Mycoplasma contamination in a sample. Mycoplasma is a common contaminant in cell cultures and can affect AAV quality, so confirming its absence is important.
- Capillary Electrophoresis (CE) is a technique used to analyze the integrity of the AAV genome. CE can detect the presence of truncated or rearranged AAV genome fragments, which can affect AAV potency and safety.
- SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and silver stain are methods for analyzing the size and purity of the AAV capsid protein. These methods can detect the presence of impurities and ensure that the capsid size is consistent with the expected size for the AAV serotype.
- TEM (transmission electron microscopy) and AUC (analytical ultracentrifugation) are methods for determining the proportion of empty capsids in the AAV sample. Empty capsids are undesirable as they do not contain the therapeutic payload and can reduce the efficacy of the AAV vector. Monitoring the empty capsid rate is important to ensure the potency and quality of the AAV product.
The combination of these tests allows for a comprehensive assessment of the AAV quality, purity, and safety, ensuring that the AAV produced by PackGene is suitable for use in NHP studies.
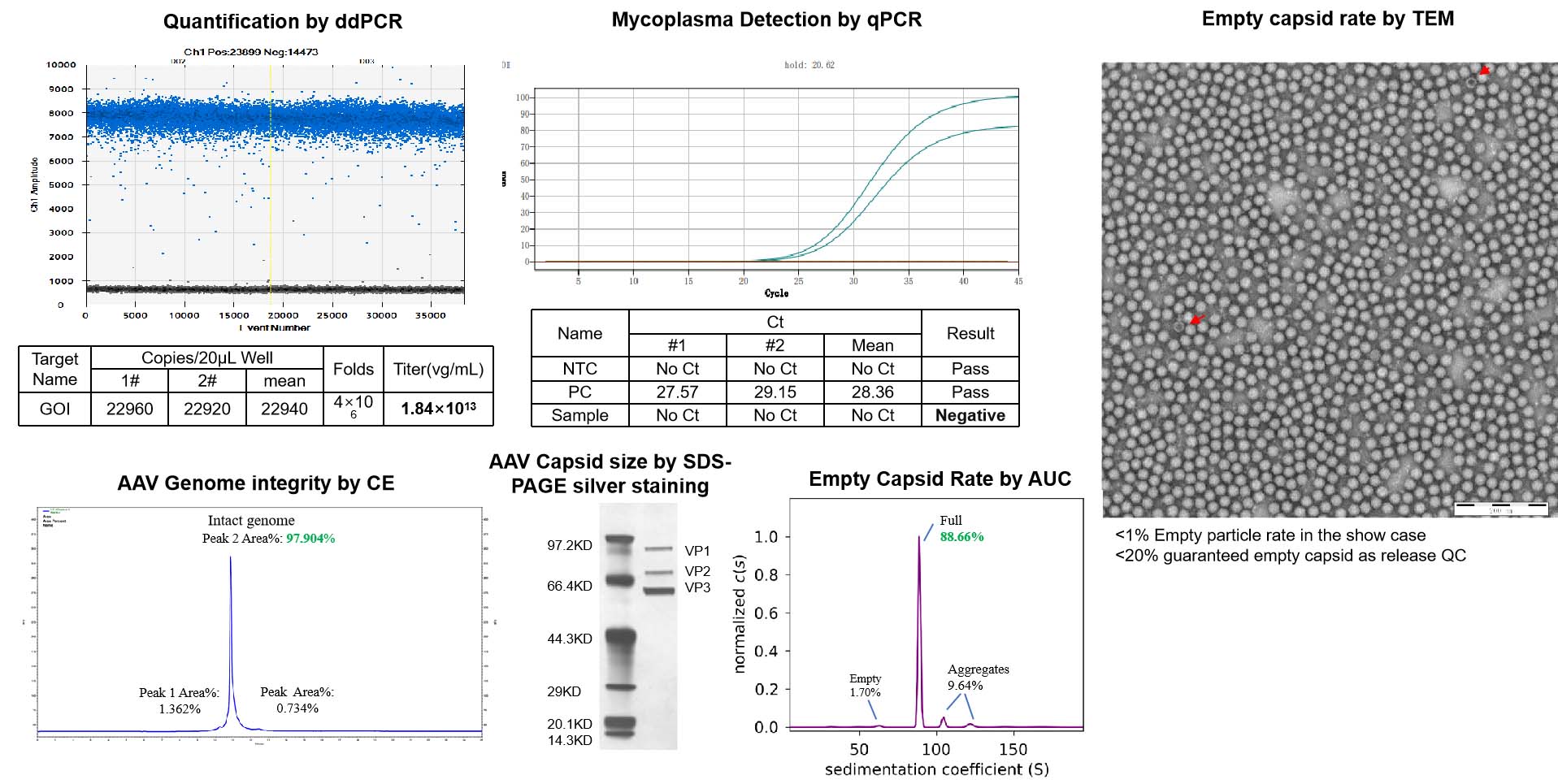
Upon receiving the AAV cis plasmid template from our customers, we conduct a double-check of the ITR integrity in addition to verifying the GOI size during clone screening before scaling up the plasmid. After the plasmid preparations are finished, another round of ITR and GOI sequencing is performed to ensure maximum fidelity. You can rest assured that the plasmid we used for NHP grade AAV are of the highest quality and integrity.
Research Grade AAV Packaging
Quality Control of Research Grade AAV Packaging Services
A variety of AAV-based QC assays have been developed by PackGene’s experienced QC team. QC tests are aimed at verification of the identity, purity, and potency of AAV viral particles for both in vitro and in vivo studies. AAV genome copies are quantified via SYBR qPCR with ATCC’s Reference AAV for titer calibration. Purity is determined by Coomassie-Blue staining.
We guarantee the endotoxin level of the AAV particles lower than 10 EU/ml. We also offer additional QC tests including ddPCR , TEM, TCID50 tittering and other QC services. Please check AAV Analytical Services to learn more.
| Category | QC Assays | QC Standard |
| Identity | Identity – GOI Sequence | Additional QC |
| Purity | SDS-PAGE Coomassie Blue Staining | Free QC |
| TEM | Additional QC | |
| AUC | Additional QC | |
| Potency & Content | qPCR | Free QC |
| ddPCR | Additional QC | |
| TCID50 | Additional QC | |
| Capsid Titer-ELISA | Additional QC | |
| Impurity | Endotoxin Test | Free QC |
| Mycoplasma Detection | Additional QC | |
| Sterility Test | Additional QC | |
| Residual Plasmid Test | Additional QC |
Standard QC
- Restriction digestion analysis using multiple endonuclease to verify the plasmids to be used for AAV packaging.
- AAV Titering by qPCR (SYBR Green with standard curve for quantification)
- Endotoxin Test by LAL assay
- AAV purity analysis by SDS-PAGE and Coomassie Staining (silver staining available upon request)
 |  |
| Note: ATCC VR-1816™ was used as the standards for AAV qPCR titering. | Legend: Lanes 2-5 and 7-1: AAV samples produced at PackGene. Lane 1,6: Marker |
Other Available Analytical Tests
HPLC purity analysis | TEM |
| AAV2-EGFP Sample produced at PackGene. The purity as analyzed by HPLC was 99%. |
HT Grade AAV Packaging
AAV Packaging – HT Grade
PackGene’s HT Grade AAV packaging service is designed to meet the needs of researchers who require high-quality AAV vectors without the premium price tag. Our streamlined process and efficient production techniques allow us to deliver high-titer AAVs at competitive prices. We exclusively offer qPCR titer measurements for this grade, ensuring precise and reliable results.
| AAV Packaging Serotypes | Guaranteed Yield (GC)* | Lead Time (Business Days) |
| Normal-yield AAV Serotypes | 2E11-2E13 | 7-14 |
| Low-yield AAV Serotypes (AAV4, 6, etc.) | 2E11-1E13 | 7-14 |
Key Features of Our HT Grade AAV Packaging Service:
- Rapid Turnaround Time: Experience expedited AAV packaging process and delivery in as fast as 7 business days.
- Cost-Effective Solution: Enjoy affordable pricing without compromising quality.
- High-Titer AAVs: Achieve optimal gene expression with our high-titer vectors.
- Reliable Quality Control: Rigorous testing ensures purity, potency, and safety.
- Flexible Customization: Tailor your AAV vectors to your specific research needs.
HT Grade AAV Packaging Protocol:
AAV Production Workflow:
- Day1: Bacteria preparation
- Day2: Plasmid preparation & enzyme digestion verification
- Day5-7: Co-tranfection in Hek293T cells
- Day8: Crude purification
- Day9: Concentration and Ultracentrification
- Day10: QC test (QPCR, endotoxinremoval, SDS-PAGE)
- Day11: QA Review and Release
1μg plasmid from customer or made by PackGene → Bacterial transformation → Plasmid preparation → Co-transfection of 293T cells → Crude purification → Concentration → Ultra-centrifuge iodixanol density-gradient → 0.2μm sterile filter → qPCR titration → SDS-PAGE coomassie blue staining endotoxin testing → AAV certification AAV QC reports
Packgene AAV Packaging Service Details
| PackGene AAV Packaging Service | |||
|---|---|---|---|
| Research Grade | NHP Grade | HT Grade | |
| Application | Cell culture, small animal | Small animal or non-human primates | Cell culture |
| Scale | 2E+12 ~ 8E+14 GC | 1E+13 ~ 1E+16 | 2E+11 ~ 2E+13 GC |
| Lead time | Start from 12 business days | Start from 17 business days | 7-14 business days |
| QC test | qPCR, SDS-PAGE Coomassie Blue Staining, endotoxin LAL, plasmid restriction enzyme digestion | ddPCR, SDS-PAGE Silver Staining, endotoxin LAL, plasmid restriction enzyme digestion | qPCR |
| Performance Comparison | |||
| Titration | qPCR, Titer meet requirements | ddPCR, Titer meet requirements | qPCR, Titer meet requirements |
| AAV purity analysis | SDS-PAGE, Coomassie Blue Staining, Capsid band size meet reference | SDS-PAGE, Silver Staining, Capsid band size meet reference, Capsid band size meet reference | – |
| Endotoxin Test | LAL, <10EU/mL | LAL, <1EU/ml (endotoxin removal may reach <0.2EU/ml) | – |
| Mycoplasma | – | qPCR, Negative | – |
| Bioburden | – | 48 hr, no growth | – |
| AAV Genome integrity | – | Capillary Electrophoresis, report value | – |
| Empty Capsid | TEM, guaranteed empty capsid rate <30% for common serotypes | TEM, guaranteed empty capsid rate <20% for common serotypes | – |
| TEM images | 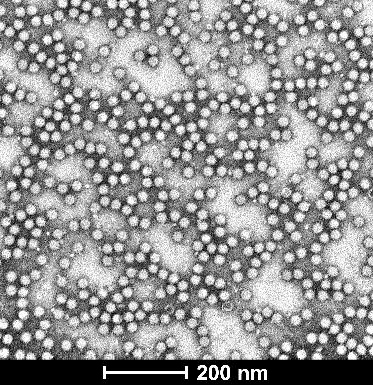 | 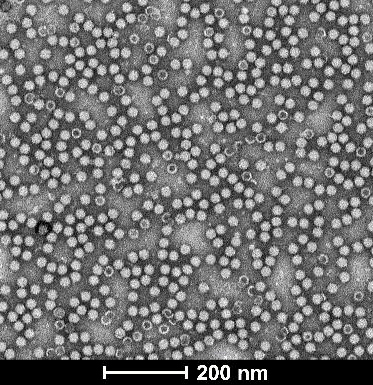 | 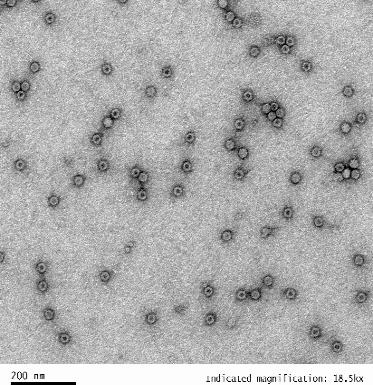 |
Performance Data
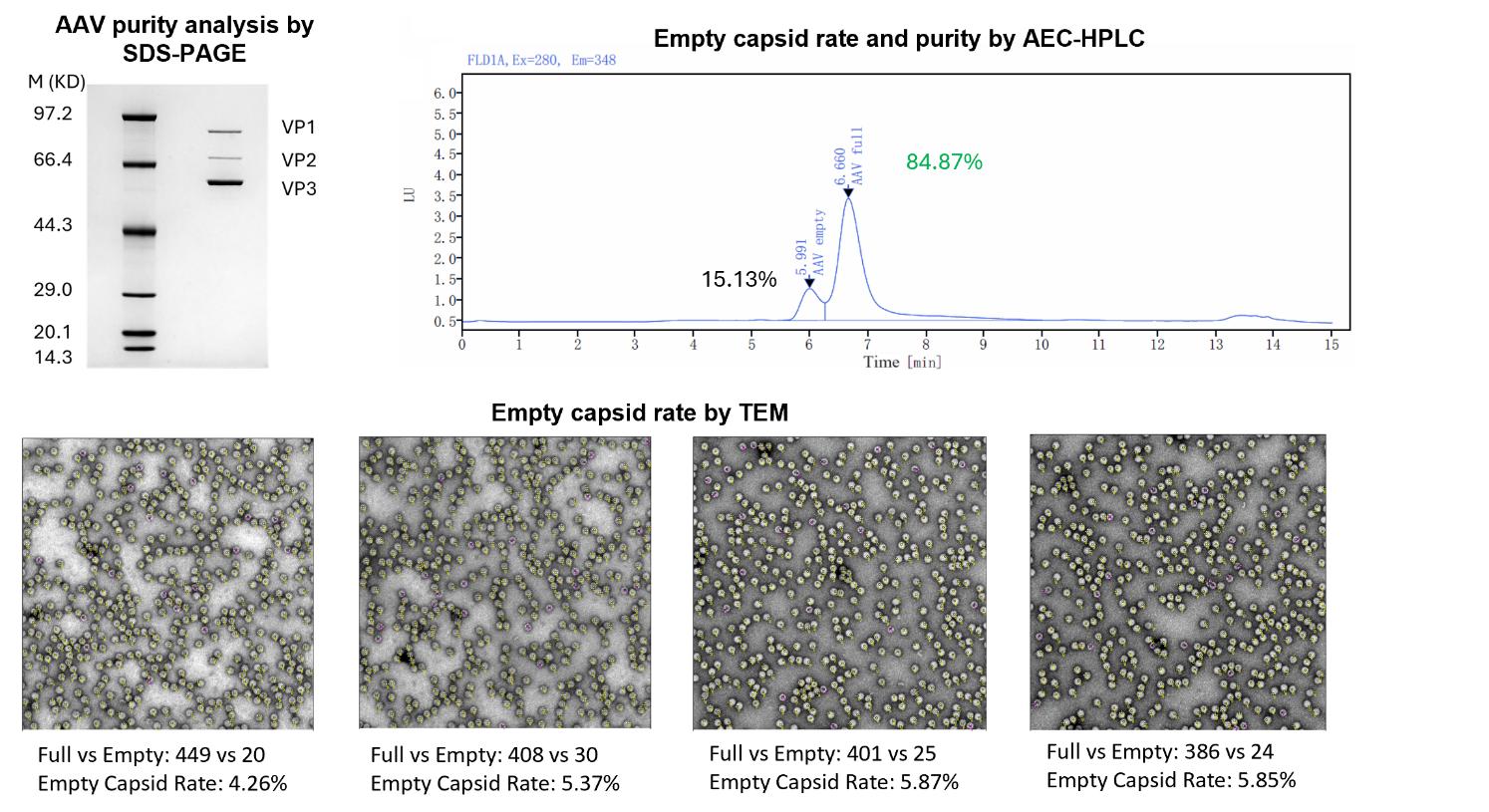
Storage Requirement:
- Store the virus at -80°C, take it out when needed, and place it on ice during operation. Fast Service must be used within 24 hours.
- Calculate your expected usage in advance and PackGene will aliquot your virus according to your pre-determined requirements. This can help avoid unnecessary thawing and re-freezing after receiving your AAVs since freeze-thaw cycles influence virus viability. If aliquoting is required, it is recommended to use PCR tubes with siliconized inner walls, or special virus preservation tubes with low protein binding rates.
- Thaw your virus aliquots in an ice bath immediately before use.
- Dilute with PBS or PBS / 0.001% F-68 if needed
Case Study
Case 1
Case 2
Case 3
Case 4

- Spinal muscular atrophy with myoclonic epilepsy (SMA-PME, ASAH1 gene defect)
- Single dose, intrathecal and intracerebroventricular injection.
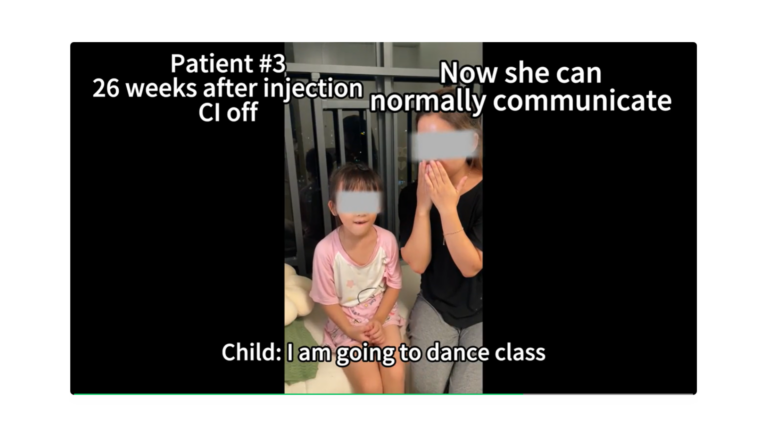
- OTOF biallelic mutations cause severe hearing impairment or complete hearing loss.
- Single dose, cochlear injection.
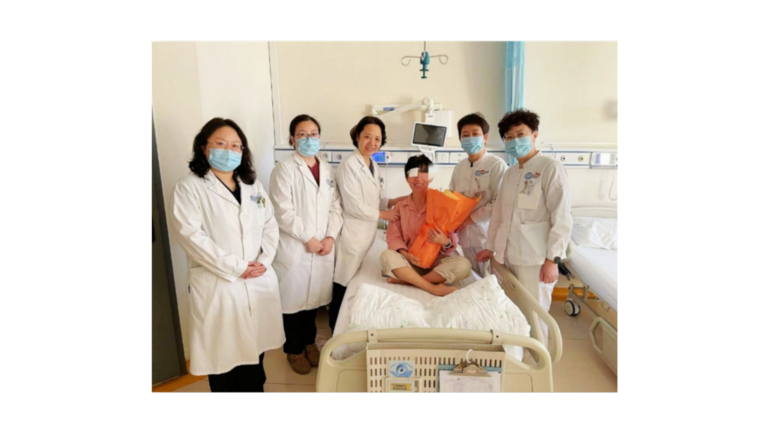
- Advanced blindness caused by retinitis pigmentosa.
- Single dose, intraocular intravitreal injection.

- Amyotrophic lateral sclerosis (ALS).
- Single dose, intrathecal injection.
Want To Inquire About The Services?
Contact Us

THE ATLANTIS BIOSCIENCE DIFFERENCE Discover Translational Solutions To Advance From Bench to Bed
GET SUPPORT Whenever You Need It

QUESTIONS IN YOUR MIND?
Connect With Our Technical Specialist.

KNOW WHAT YOU WANT?
Request For A Quotaiton





