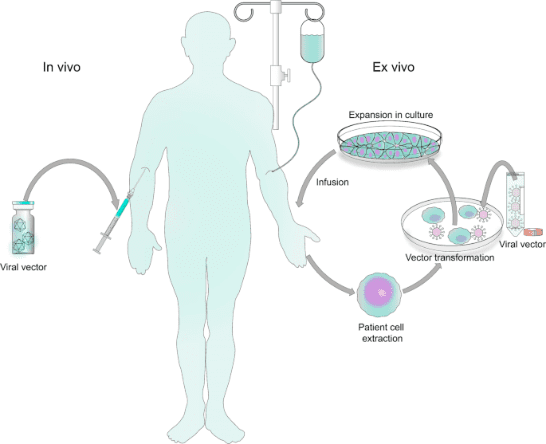
| CAR T Cells-based Therapy |
KYMRIAH
(2017) | Autologous CAR T cells expressing anti-CD19 CAR transgene via lentiviral vector | Novartis Pharmaceuticals Corporation | For adult patients with certain kinds of relapsed or refractory follicular lymphoma |
YESCARTA
(2017) | Autologous CAR T cells expressing anti-CD19 CAR transgene via retroviral vector | Kite Pharma, Inc. | For adult patients with certain kinds of relapsed or refractory large B-cell lymphoma |
TECARTUS
(2020) | Autologous CAR T cells expressing anti-CD19 CAR transgene via retroviral vector | Kite Pharma, Inc. | For adult patients with certain kinds of relapsed or refractory mantle cell lymphoma |
ABECMA
(2021) | Autologous CAR T cells expressing anti-B-cell maturation antigen (BCMA) CAR transgene via lentiviral vector | Celgene Corporation, a Bristol-Myers Squibb Company | For adult patients with certain kinds of relapsed or refractory multiple myeloma |
BREYANZI
(2021) | Autologous CAR T cells expressing anti-CD19 CAR transgene via lentiviral vector | Juno Therapeutics, Inc., a Bristol-Myers Squibb Company | For adult patients with certain kinds of large B-cell lymphoma |
CARVYKTI
(2022) | Autologous CAR T Cells expressing anti-BCMA CAR transgene via lentiviral vector | Janssen Biotech, Inc. | For adult patients with certain kinds of relapsed or refractory multiple myeloma |
| Immune Cells-based Therapy |
PROVENGE
(2010) | Autologous antigen-presenting cells (APC) activated with prostatic acid phosphatase (PAP)- granulocyte-macrophage colony-stimulating factor (GM-CSF), PAP-GM-CSF | Dendreon Corp. | For treatment of certain types of prostate cancer |
| AMTAGVI (2024) | Autologous tumour-derived T cells | Iovance Biotherapeutics, Inc. | For adult patients with unresectable or metastatic melanoma previously treated with a PD-1 blocking antibody, and if BRAF V600 mutation positive, a BRAF inhibitor with or without a MEK inhibitor |
| Hematopoietic Progenitor Cell-based Therapy |
HEMACORD
(2011) | Allogenic hematopoietic progenitor cells; Cord blood | New York Blood Center | For hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system |
DUCORD
(2012) | Allogenic hematopoietic progenitor cells; Cord blood | Duke University School of Medicine | For hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system |
HPC, Cord Blood
(2012) | Allogenic hematopoietic progenitor cells; Cord blood | Clinimmune Labs, University of Colorado Cord Blood Bank | For hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system |
ALLOCORD
(2013) | Allogenic hematopoietic progenitor cells; Cord blood | SSM Cardinal Glennon Children’s Medical Center | For hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system |
CLEVECORD
(2016) | Allogenic hematopoietic progenitor cells; Cord blood | Cleveland Cord Blood Center | For hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system |
HPC, Cord Blood
(2016) | Allogenic hematopoietic progenitor cells; Cord blood | LifeSouth Community Blood Centers, Inc. | For hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system |
HPC, Cord Blood
(2016) | Allogenic hematopoietic progenitor cells; Cord blood | Bloodworks | For hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system |
| HPC, Cord Blood (2018) | Allogenic hematopoietic progenitor cells; Cord blood | MD Anderson Cord Blood Bank | For hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system |
OMISIRGE
(2023) | Nicotinamide modified allogenic hematopoietic progenitor cell | Gamida Cell Ltd. | For use in adults and pediatric patients 12 years and older with hematologic malignancies |
| Other Cell-based Therapy |
LAVIV
(2011) | Autologous dermal fibroblasts | Fibrocell Technologies | For improvement in the appearance of moderate to severe nasolabial fold wrinkles in adults |
GINTUIT
(2012) | Cellularised scaffold of allogeneic keratinocytes and dermal fibroblasts, human extracellular matrix proteins, and bovine collagen | Organogenesis Inc. | For topical application to a surgically created vascular wound bed in the treatment of mucogingival conditions in adults |
MACI
(2016) | Cellularised scaffold of autologous chondrocytes on porcine collagen membrane | Vericel Corp. | For repair of cartilage defects of the knee in adults |
STRATAGRAFT
(2021) | Cellularised scaffold of allogenic keratinocytes and dermal fibroblasts in murine collagen | Stratatech Corporation | For adult patients with some types of thermal burns |
RETHYMIC
(2021) | Allogenic thymus tissue | Enzyvant Therapeutics GmbH | For immune reconstitution in pediatric patients with congenital athymia |
LANTIDRA
(2023) | Allogenic pancreatic islet cells from deceased donor | CellTrans Inc. | For adult patients with certain types of type I diabetes |
| CD34+ HSC-based Gene Therapy |
SKYSONA
(2022) | Autologous CD34+ hematopoietic stem cells (HSC) engineered with Lenti-D vectors expressing the ABCD1 gene | bluebird bio, Inc. | For patients with a kind of cerebral adrenoleukodystrophy |
ZYNTEGLO
(2022) | Autologous CD34+ HSC engineered with a BB305 lentivirus encoding β-globin gene (βA-T87Q-globin gene) | bluebird bio, Inc. | For adult and pediatric patients with certain types of ß-thalassemia |
CASGEVY
(2023) | Autologous CD34+ HSC engineered with electroporation of CRISPR/Cas9 RNP complexes to decrease expression of BCL11A to increase foetal hemoglobin production | Vertex Pharmaceuticals, Inc. | For patients 12 years and older with certain kinds of sickle cell disease |
LYFGENIA
(2023) | Autologous CD34+ HSC engineered with a BB305 lentivirus encoding β-globin gene (βA-T87Q-globin gene) | bluebird bio, Inc. | For patients 12 years of age or older with certain kinds of sickle cell disease |
| LENMELDY (2024) | Autologous CD34+ HSC engineered with a lentivirus encodingthe human arylsulfatase A (ARSA) gene | Orchard Therapeutics (Europe) Limited | For children with certain kinds of metachromatic leukodystrophy (MLD) |
| Adenoviral/Adeno-associated viral-based Gene Therapy |
LUXTURNA
(2017) | A recombinant adeno-associated virus (AAV2) containing a transgene encoding RPE65 | Spark Therapeutics, Inc. | For patients with RPE65-mutation-associated retinal dystrophy |
ZOLGENSMA
(2019) | A recombinant adeno-associated virus (AAV9) containing a transgene encoding the human survival motor neuron (SMN) protein | Novartis Gene Therapies, Inc. | For pediatric patients with certain types of spinal muscular atrophy |
ADSTILADRIN
(2022) | A recombinant adenovirus containing a transgene encoding the human interferon alfa-2b (IFNα2b) | Ferring Pharmaceuticals A/S | For adult patients with certain types of bladder cancer |
HEMGENIX
(2022) | A recombinant adeno-associated virus (AAV) containing a transgene encoding the gain-of-function Padua variant of human Factor IX (variant R338L) | CSL Behring LLC | For adult patients with certain kinds of Hemophilia B |
ELEVIDYS
(2023) | A recombinant adeno-associated virus (AAVrh74) containing a transgene encoding the micro-dystrophin protein | Sarepta Therapeutics, Inc. | For pediatric patients with certain types of Duchenne muscular dystrophy |
ROCTAVIAN
(2023) | A recombinant adeno-associated virus (AAV5) containing a transgene encoding the B-domain deleted SQ form of the human coagulation factor VIII (hFVIII-SQ) | BioMarin Pharmaceutical Inc. | For adult patients with certain types of hemophilia A |
| Oncolytic Viral-based Gene Therapy |
IMLYGIC
(2015) | Recombinant HSV-1 expressing huGM-CSF to promote an anti-tumour immune response | BioVex, Inc., a subsidiary of Amgen Inc. | Local treatment in patients with certain unresectable melanoma lesions |
VYJUVEK
(2023) | Recombinant HSV-1expressing collagen type VII gene (COL7) | Krystal Biotech, Inc. | Topical treatment of certain wounds in patients with dystrophic epidermolysis bullosa with mutations in the COL7A1 gene |