- Your cart is empty
- Continue Shopping

FBS, FCS and Calf Serum: What’s the difference?
What is Bovine Serum? In cell culture, selecting the right serum is crucial for the success of your experiments.Bovine serum, derived from the liquid portion
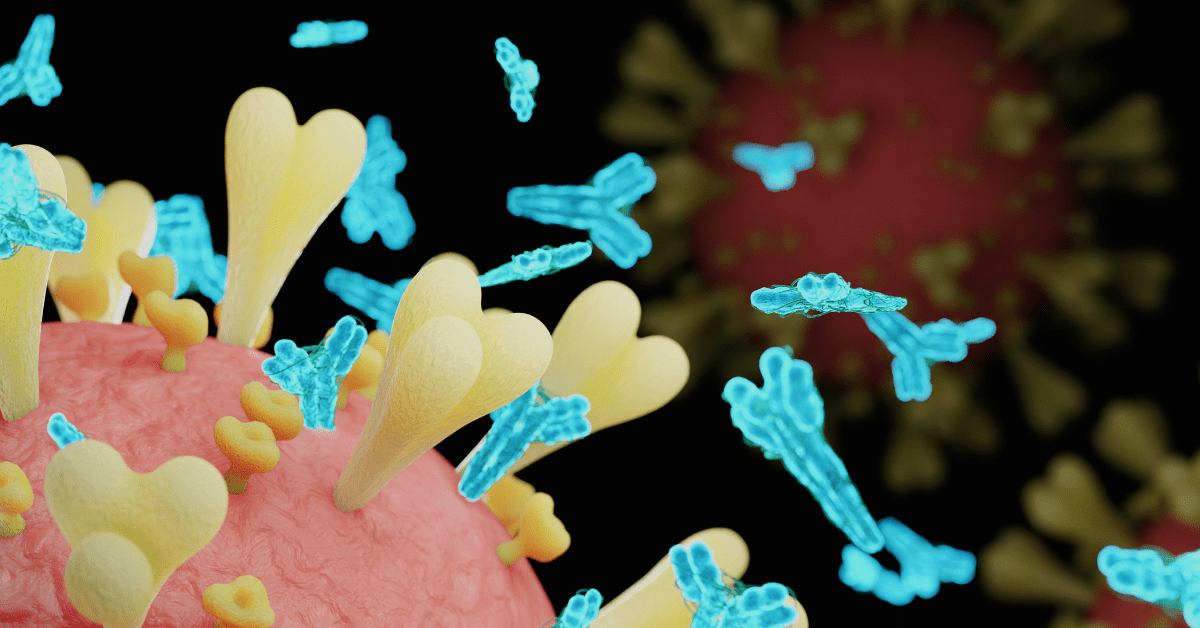
An antibody, also known as an immunoglobulin, is a specialized protein produced by the immune system in response to the presence of foreign substances called antigens. The primary function of antibodies is to recognize, bind to, and neutralize antigens such as bacteria, viruses, and other harmful substances.
By binding to antigens, antibodies can neutralize their effects, mark them for destruction by other immune cells, or activate various immune responses to eliminate the invading pathogens. Additionally, antibodies play essential roles in immune memory and protection against recurrent infections.
Antibodies are essential tools in research, enabling scientists to detect specific proteins or molecules in biological samples. Used in techniques like Western blotting, immunohistochemistry, or flow cytometry, these targeted agents provide precise insights into cellular components and disease mechanisms. Their specificity facilitates advancements in medicine and biotechnology, making antibodies invaluable for scientific exploration and innovation.
The structure of an antibody is a Y-shaped molecule of ~150 kDa, composed of four polypeptide chains: two identical heavy chains and two identical light chains. Each heavy chain is ~50 kDa, while each light chain is ~25 kDa. The two heavy chains are linked to each other by disulfide bonds and each heavy chain is linked to a light chain by a disulfide bond.
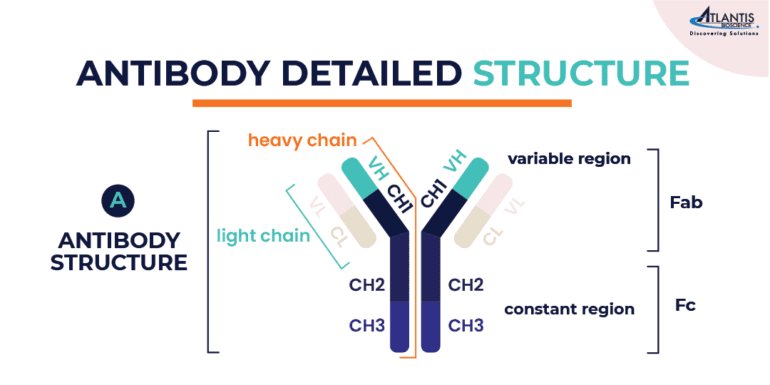
Each heavy and light chain is composed of a series of similar domains. The light chain is composed of two domains (VL, CL) while the heavy chain of IgG antibody contains four (VH, CH1, CH2, CH3). The tips of the Y-shaped structure contain variable regions (V), which are specific for each antibody and allow it to recognize and bind to a particular antigen. These variable regions are highly variable in sequence and structure, enabling antibodies to interact with a wide variety of antigens. The lower part of the Y-shaped antibody structure contains constant regions (C). These regions determine the antibody’s isotype (e.g., IgG, IgM, IgA, IgE, IgD) and its specific functions, such as binding to immune cells or activating other components of the immune system.
The antibodies can also be segmented into two regions: the Fab, or antigen-binding fragment, and the Fc, or crystallizable fragment. The Fab fragment is composed of the light chain and part of the heavy chain where the variable domains are, while the Fc region is made up of the rest of the heavy chain composed of the constant domains.
The capability of antibodies to recognize and bind to specific proteins is invaluable in research. This specificity enables scientists to pinpoint particular proteins of interest. Here are some common applications of antibodies in research:
This technique involves separating proteins based on size through gel electrophoresis and then transferring them onto a membrane. Primary antibodies specific to the protein of interest are then used to bind to the protein on the membrane. Then, secondary antibodies are added to allow the protein to be detected by chemiluminescence or fluorescence. Western blotting is frequently used to provide information about the protein size and abundance.
Check out Atlantis Bioscience Western Blotting Solutions.
Immunocytochemistry (staining of cells) and Immunohistochemistry (staining of tissues) both utilize antibodies to provide visual details about protein abundance, distribution, and localization. These could be visualized using antibodies conjugated with fluorescent tags (i.e. immunofluorescence) or enzyme-linked tags (i.e. chromogenic detection), followed by microscopy techniques.
Check out Atlantis Bioscience Cell Imaging Solutions.
Antibodies tagged with fluorescent markers are employed in flow cytometry to analyze and categorize cells according to specific surface markers or intracellular proteins. One notable advantage of flow cytometry is its capacity to utilize numerous fluorescent signals—sometimes exceeding a dozen—to concurrently assess both the relative and absolute expression levels of multiple proteins within individual cells and across different samples. This method proves invaluable for investigating cell populations, immune reactions, and cellular signaling pathways.
Check out Atlantis Bioscience Flow Cytometry Solutions.
ELISA is a widely used method for detecting and quantifying specific proteins or antibodies in a sample. In this assay, a target molecule is immobilized on a solid surface, such as a microplate. After blocking non-specific binding sites, a specific antibody or antigen labeled with an enzyme is added, which binds to the target molecule. Following a series of washing steps to remove unbound substances, a substrate is added, leading to a color change or fluorescent signal proportional to the amount of the target molecule present in the sample.
Antibodies are used to isolate specific proteins or protein complexes from a complex mixture of proteins. In this method, antibodies bound to microscopic beads grab the protein of interest and pull it out of the solution. Once the unbound protein is washed away, the protein of interest is dissociated from the bead and the antibody using a low-pH buffer. Then, the purified protein can be used in experiments to compare the relative amount of protein present in a series of samples, such as Western blots or ELISAs, or for other experiments to study protein-protein interactions.
Monoclonal antibodies (mAbs) represent a cutting-edge advancement in biotechnology, harnessing the precision of the immune system to create tailored therapeutic and research tools. The hybridoma technology is the most widely used method for generating mAbs. Hybridomas (fusion of a B cell with a myeloma cell) are generated to produce a continuous supply of mAbs specific to the antigen. These mAbs are designed to selectively bind to specific molecules involved in diseases, such as cancer or autoimmune disorders.
In conclusion, learning about antibodies gives us a clearer picture of how these important molecules work. Understanding their structure helps us see how they interact with other things in the body. From this foundation, we can see how researchers use antibodies in many ways, like diagnosing illnesses and creating new treatments. Antibodies play a big role in both our health and ongoing medical research.
References
Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. The structure of a typical antibody molecule. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27144/

Connect With Our Technical Specialist.

Request For A Quotaiton

What is Bovine Serum? In cell culture, selecting the right serum is crucial for the success of your experiments.Bovine serum, derived from the liquid portion
Mesenchymal stem cells (MSCs) hold immense promise in regenerative medicine due to their multilineage differentiation potential. However, large-scale expansion of these therapeutic cells can be
Extracellular vesicles (EVs) are revolutionising our understanding of cell-to-cell communication. These ubiquitous messengers encased in a lipid bilayer membrane, are nano-sized packages actively released by

Contact our Customer Care, Sales & Scientific Assistance

Consult and asked questions about our products & services

Documentation of Technical & Safety Data Sheet, Guides and more..