Each cell type has a different function and lineage which affects how cells respond to each other and to their environment. But how can we study these unique cell types in depth?
Single-cell analysis is a commonly used method to understand the cell’s characteristics and behaviour with other cells. Before we go into single-cell analysis, it is essential to isolate the unique cell from the whole tissue or cell sample. This process is often the biggest challenge in single-cell studies.
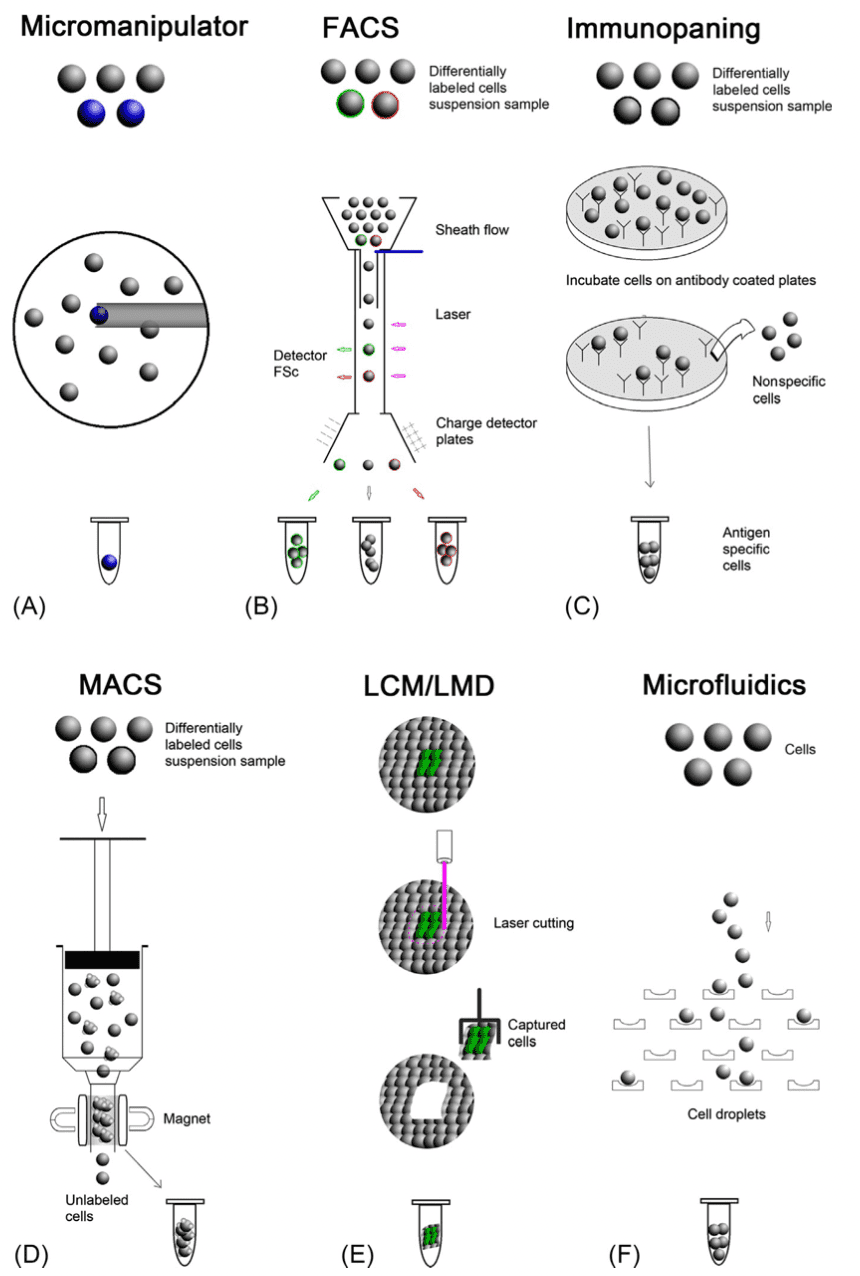
Single-cell isolation can be done through various single-cell isolation techniques. In this article, we will explain the seven common and popular techniques used for single-cell isolation.
1. Micromanipulator
Advantage: Intact live tissue
Limitation: Requires high skill level and low throughout
Single-cell isolation can be performed using a micromanipulator. Manual micromanipulation is simple and robust and involves a microscope and a micropipette to pick up the desired cell manually. It is often used to isolate embryo cells or live culture cells. While this technique is simple and fast, it can only process a small number of cells at a time as it requires professional skills, and cannot be used with complex cells. To improve the efficiency of micromanipulation, robotic micromanipulators were developed. Robotic micromanipulation uses computer vision-based robotic systems to select specific cells. Many robotic micromanipulators have been introduced to date. This includes an automated micropipette that is coupled with image analysis to allow the screening of cells that express desired proteins.
2. Fluorescence-Activated Cell Sorting
Advantages: Multiple parameters, high throughput, highly compatible with workflow
Limitation: A large amount of material, dissociated cells, requires high skill levels
Fluorescence-Activated Cell Sorting (FACS) is a widely used technique for isolating single cells. The target cells are labelled with fluorescent markers, which are fluorophore-conjugated monoclonal antibodies that recognize specific markers on the target cells. As the cell suspension flows through the cytometry, cells are exposed to lasers which allow the fluorescence detectors to detect the fluorescent marker on the cells. Although FACS is commonly used in many applications, there are several disadvantages, such as a large starting number of cells (more than 10,000 cells), the use of dissociated cells, and the damage to the viability of the sorted cells due to the rapid flow in the machine.
3. Microfluidics
Advantages: High throughput, high specificity and sensitivity, low sample consumption, time and cost-effectiveness
Limitation: Dissociated cells, require high skill levels
As compared to other single-cell sorting methods like FACS or MACS, microfluidic is widely used in clinical diagnosis. Microfluidics provides precise fluid control, low sample consumption, device miniaturisation and low analysis cost. Various microfluidic chip designs have been developed for isolating single cells. As microfluidics does not require labelling, single-cell isolation using microfluidics is based on the intrinsic physical properties of cells, such as cell size, shape, density, deformability, electric polarizability/impedance, and other hydrodynamic properties.
4. Immunopanning
Advantages: High specificity
Limitation: Expensive and low throughput
Immunostaining (PAN) is a cell-sorting technique based on cell-type-specific antigens. This procedure involves incubating the dissociated cells with an antibody-coated plate to capture the specific antigens on the cells. The target cells bind to the antibody, and the unbound cells are washed off. This method is expensive as a specific antibody is required for a specific cell type, and selecting an effective antibody can be time-consuming.
5. Magnet-Activated Cell Sorting
Advantage: High specificity, cost-effective
Limitation: Low throughput, dissociated cells, nonspecific cell capture
Magnet-Activated Cell Sorting (MACS) is based on conjugated magnetic beads to bind to specific proteins on the target cell. When a cell suspension is placed in a magnetic field, the magnetic beads will attract the target cells, while the non-attracted cells will be washed out. The remaining cells will then be subsequently eluted out. MACS has two modes of separation – positive or negative. The positive separation technique uses coated magnetic beads to attract the desired cells. The labelled cells are the cells of interest while the unlabelled cells are discarded.
On the other hand, in the negative separation technique, if species-specific substances are unavailable, a cocktail of antibodies can be used to coat the untreated cells. Unlabelled cells will be captured while the labelled cells are discarded. The final purity of the isolated cells in MACS depends on the specificity and the affinity of the antibodies. One of the major disadvantages of MACS is the non-specific cell capture due to the adsorption of the background cells to the magnetic beads. Compared to FACS, MACS is limited in the immunomagnetic techniques, as it can only separate cells into positive and negative populations
6. Laser Microdissection
Advantages: Intact fixed and live tissue and single-cell control
Limitation: Low throughput, requires high skill levels, contaminated by neighbouring cells
Laser microdissection (LCM) is a technique that isolates cells from a tissue sample. It is suitable for solid tissue analysis. It involves identifying the tissue under a microscope and marking the desired section, followed by using a laser beam to cut the selected area to isolate the cells. LCM does not require tagging, but the technique is time-consuming and expensive and requires highly skilled personnel. Cells are sometimes damaged and there may be a high chance of contamination by the neighbouring cells.
7. Limiting Dilution
Advantages: Simple, cost-effective, reproducible
Limitation: Lack of control, requires downstream processes to prove the presence of single cells
Limiting dilution is a method that uses the dilution of the cell suspension to isolate single cells. The cell suspension is diluted to the point that there is only one cell in a single microwell. It is a technique often used to produce monoclonal cell cultures, for antibody production through hybridomas, and for single-cell-based assays.
Overcoming initial single-cell isolation challenges with new technology
According to Dr. Marian Rehak, VP of Research and Development at Sphere Fluidics, although there are several techniques for single-cell isolation, the initial isolation and culture of a single cell are one of the major challenges. It is difficult to culture freely as cells do not live on their own. They are influenced by their environment and cell-to-cell interaction, which is absent in an artificial environment. This will cause an unnatural response and may induce unintended cell stress, changes in cell behaviour, cell viability and the change of cell profile.
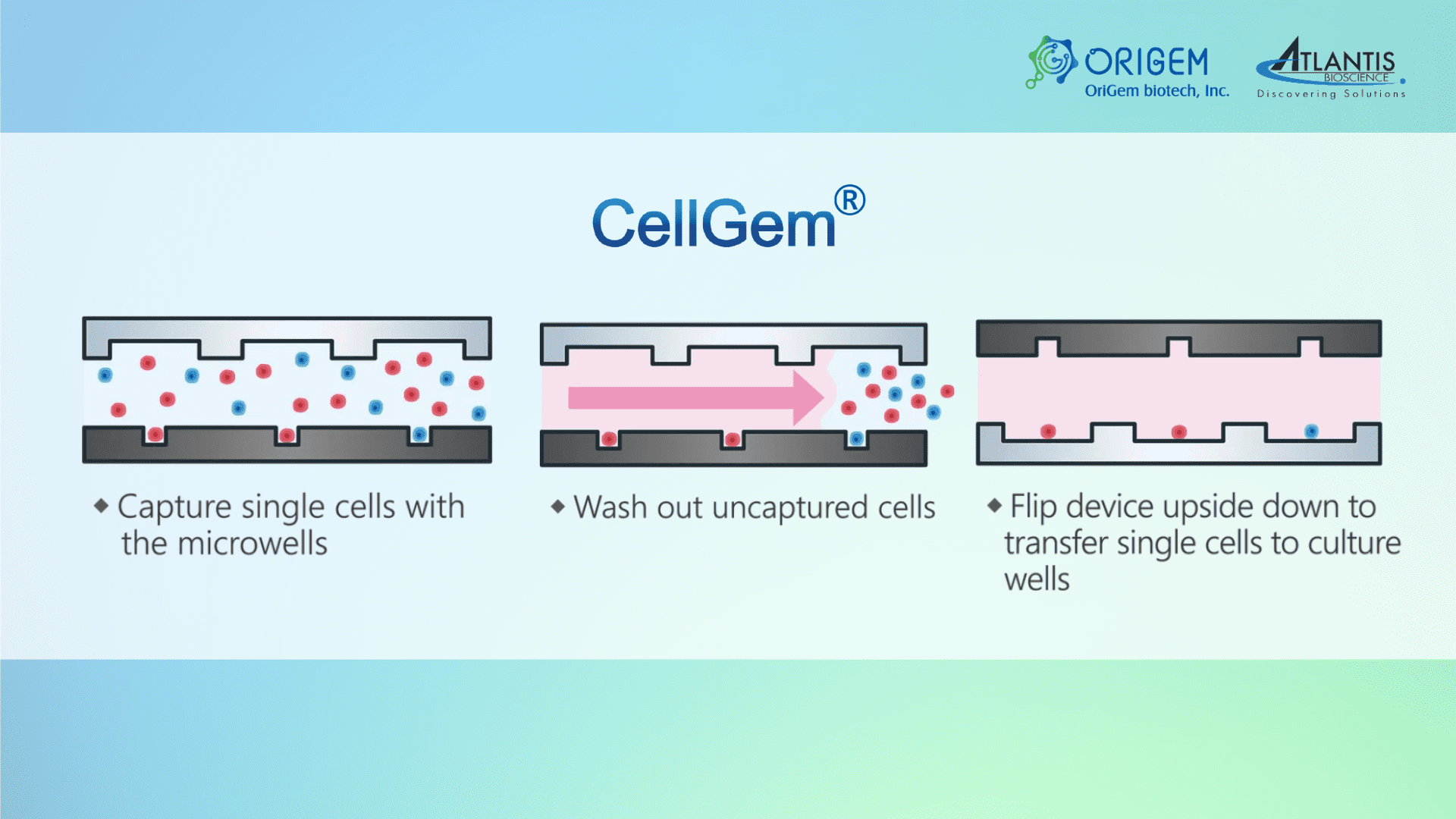
Origem is a biotech startup company that has developed an innovative single-cell isolation & culture platform. CellGem is a simple and smart single-cell isolation and culture device. It is a high throughput platform that uses microfluidics technology, providing high single-cell efficiency and high cell viability, with low sample consumption.
CellGem platform is composed of microwells to capture single cells and a corresponding array of culture wells for long-term culture of the single cells. This can be used for stable cell line development and cell heterogeneity studies.
Interested to find out more about CellGem’s single-cell isolation technology?