High-Quality cGMP Heparin Sodium Salt
Akron Biotech’s Heparin Sodium Salt is a premium-grade anticoagulant manufactured under stringent cGMP guidelines. Backed by a Type II Master File, this product is ideal for cell and gene therapy applications.
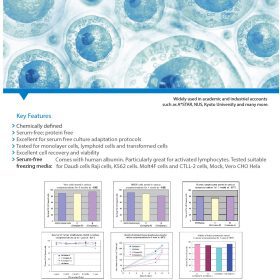
Key Features:
- cGMP Adherence: Produced, tested, and released following rigorous regulatory standards.
- Proven Efficacy: Inactivates key clotting factors for optimal cell culture conditions.
- Versatility: Suitable for a wide range of cell and gene therapy applications.
- High Purity: Meets EP standards for Heparin Sodium Salt.
- Extensive Testing: Characterized by H-NMR, HSQC, and IR spectroscopy.
- Sustainable Sourcing: Raw material sourced from inspected and registered animal facilities.
Manufacturing Excellence:
- Leveraging pharmaceutical-grade production processes.
- Validated inactivation of viruses and removal of impurities.
- Sterile microfiltration before drying and homogenization.
Quality Assurance:
- Adherence to USP , EP 5.2.12, and ISO 20399:2022 standards.
- Rigorous testing to guarantee product quality and consistency.
Stability and Storage:
- Stable for 5 years when stored below 40°C, protected from light and humidity.
Unlock the potential of your research with Akron Biotech’s Heparin Sodium Salt.