Mabuterol is a selective agonist of β2 adrenoreceptor with no beta 1-stimulation. Mabuterol inhibited the positive inotropic effect of isoprenaline at 10(-7) g/ml and decreased the maximum driving frequency at 3 X 10(-6) g/ml. Mabuterol was 3 times more potent in relaxing the isolated rat uterus, but 700 times less potent than isoprenaline in relaxing the rabbit jejunum. Mabuterol (p.o.) depressed the intestinal propulsion and was equipotent to isoprenaline and 2.5 times less potent than salbutamol.
[accordions]
[accordion title= “Chemical Properties“]
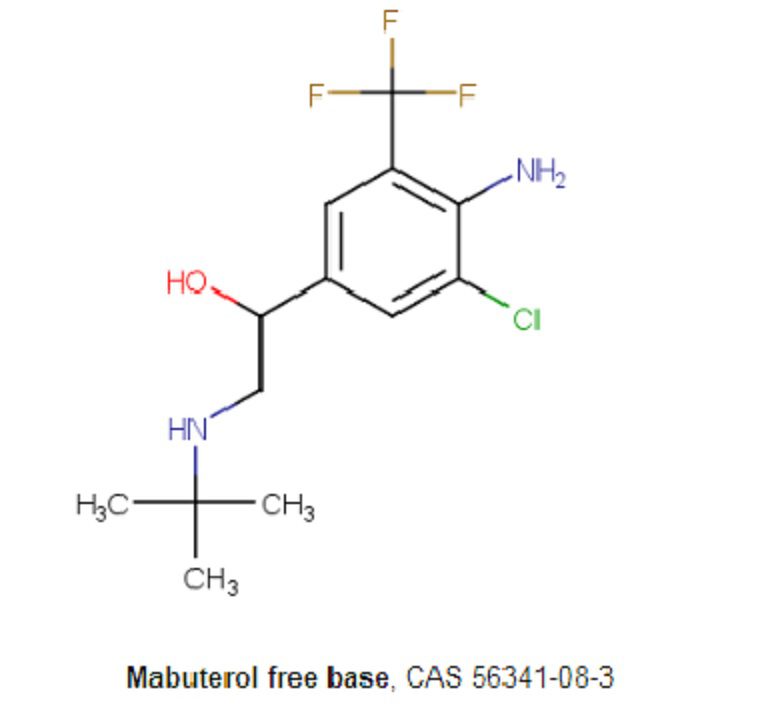
| Synonyms | Mabuterol, PB 868Cl |
| Molecular Weight | 310.74 |
| Formula | C13H18ClF3N2O |
| CAS No. | 56341-08-3 |
[/accordion]
[accordions]
[accordion title= “References and Literature“]
1. Song X, Zhao C, Dai C, Ren Y, An N, Wen H, Pan LI, Cheng M, Zhang Y. Suppression of the increasing level of acetylcholine-stimulated intracellular Ca(2+) in guinea pig airway smooth muscle cells by mabuterol. Biomed Rep. 2015 Nov;3(6):778-786. Epub 2015 Aug 4. PubMed PMID: 26623015; PubMed Central PMCID: PMC4660599.2. Lu X, Liu P, Chen H, Qin F, Li F. Enantioselective pharmacokinetics of mabuterol in rats studied using sequential achiral and chiral HPLC. Biomed Chromatogr. 2005 Nov;19(9):703-8. PubMed PMID: 16206140.3. Amemiya K, Asano T, Arika T, Nakamura M, Kudoh M. Special toxicology–physical dependence potential, antigenicity and mutagenicity–of mabuterol. Arzneimittelforschung. 1984;34(11A):1685-6. PubMed PMID: 6152162.4. Tu Y, Zhong J, Wang H, Pan J, Xu Z, Yang W, Luo Y. Synthesis of stable isotope labeled D(9) -Mabuterol, D(9) -Bambuterol, and D(9) -Cimbuterol. J Labelled Comp Radiopharm. 2016 Nov;59(13):546-551. doi: 10.1002/jlcr.3446. Epub 2016 Oct 13. PubMed PMID: 27739098.
[/accordion]













